Joint Final Report of EORTC 26951 and RTOG 9402: Phase III Trials With Procarbazine, Lomustine, and Vincristine Chemotherapy for Anaplastic Oligodendroglial Tumors
- PMID: 35731991
- PMCID: PMC9362869
- DOI: 10.1200/JCO.21.02543
Joint Final Report of EORTC 26951 and RTOG 9402: Phase III Trials With Procarbazine, Lomustine, and Vincristine Chemotherapy for Anaplastic Oligodendroglial Tumors
Abstract
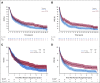
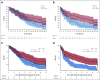
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the basis of the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.Anaplastic oligodendroglial tumors (AOTs) are chemotherapy-sensitive brain tumors. We report the final very long-term survival results from European Organization for the Research and Treatment of Cancer 26951 and Radiation Therapy Oncology Group 9402 phase III trials initiated in 1990s, which both studied radiotherapy with/without neo/adjuvant procarbazine, lomustine, and vincristine (PCV) for newly diagnosed anaplastic oligodendroglial tumors. The median follow-up duration in both was 18-19 years. For European Organization for the Research and Treatment of Cancer 26951, median, 14-year, and probable 20-year overall survival rates without versus with PCV were 2.6 years, 13.4%, and 10.1% versus 3.5 years, 25.1%, and 16.8% (N = 368 overall; hazard ratio [HR] 0.78; 95% CI, 0.63 to 0.98; P = .033), with 1p19q codeletion 9.3 years, 26.2%, and 13.6% versus 14.2 years, 51.0%, and 37.1% (n = 80; HR 0.60; 95% CI, 0.35 to 1.03; P = .063), respectively. For Radiation Therapy Oncology Group 9402, analogous results were 4.8 years, 16.5%, and 11.2% versus 4.8 years, 29.1%, and 24.6% (N = 289 overall; HR 0.79; 95% CI, 0.61 to 1.03; P = .08), with codeletion 7.3 years, 25.0%, and 14.9% versus 13.2 years, 46.1%, and 37% (n = 125; HR 0.61; 95% CI, 0.40 to 0.94; P = .02), respectively. With that, the studies show similar long-term survival even without tumor recurrence in a significant proportion of patients after first-line treatment with radiotherapy/PCV.
Trial registration: ClinicalTrials.gov NCT00002569 NCT00002840.
Conflict of interest statement
Figures
Comment in
-
Final Report of EORTC 26951 and RTOG 9402 for Anaplastic Oligodendroglial Tumors.J Clin Oncol. 2023 Feb 1;41(4):933-934. doi: 10.1200/JCO.22.01492. Epub 2022 Oct 3. J Clin Oncol. 2023. PMID: 36191285 No abstract available.
References
-
- van den Bent MJ, Carpentier AF, Brandes AA, et al. : Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: A randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol 24:2715-2722, 2006 - PubMed
-
- Cairncross G, Berkey B, Shaw E, et al. : Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol 24:2707-2714, 2006 - PubMed
-
- van den Bent MJ, Brandes AA, Taphoorn MJ, et al. : Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: Long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol 31:344-350, 2013 - PubMed
-
- Cairncross JG, Ueki K, Zlatescu MC, et al. : Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst 90:1473-1479, 1998 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

