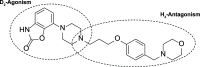
Binding of the Dual-Action Anti-Parkinsonian Drug AG-0029 to Dopamine D2 and Histamine H3 Receptors: A PET Study in Healthy Rats
- PMID: 35732005
- PMCID: PMC9257755
- DOI: 10.1021/acs.molpharmaceut.2c00121
Binding of the Dual-Action Anti-Parkinsonian Drug AG-0029 to Dopamine D2 and Histamine H3 Receptors: A PET Study in Healthy Rats
Abstract
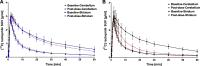
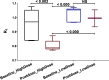
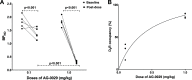
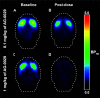
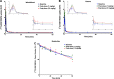
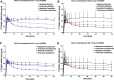
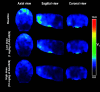
Introduction: Parkinson's disease (PD) is a neurodegenerative disorder characterized by motor dysfunction and a diverse range of nonmotor symptoms. Functional relationships between the dopaminergic and histaminergic systems suggest that dual-action pharmaceuticals like AG-0029 (D2/D3 agonist/H3 antagonist) could ameliorate both the motor and cognitive symptoms of PD. The current study aimed to demonstrate the interaction of AG-0029 with its intended targets in the mammalian brain using positron emission tomography (PET). Methods: Healthy male Wistar rats were scanned with a small-animal PET camera, using either the dopamine D2/D3 receptor ligand [11C]raclopride or the histamine H3 receptor ligand [11C]GSK-189254, before and after treatment with an intravenous, acute, single dose of AG-0029. Dynamic [11C]raclopride PET data (60 min duration) were analyzed using the simplified reference tissue model 2 (SRTM2) with cerebellum as reference tissue and the nondisplaceable binding potential as the outcome parameter. Data from dynamic [11C]GSK-189254 scans (60 min duration) with arterial blood sampling were analyzed using Logan graphical analysis with the volume of distribution (VT) as the outcome parameter. Receptor occupancy was estimated using a Lassen plot. Results: Dopamine D2/3 receptor occupancies in the striatum were 22.6 ± 18.0 and 84.0 ± 3.5% (mean ± SD) after administration of 0.1 and 1 mg/kg AG-0029, respectively. In several brain regions, the VT values of [11C]GSK-189254 were significantly reduced after pretreatment of rats with 1 or 10 mg/kg AG-0029. The H3 receptor occupancies were 11.9 ± 8.5 and 40.3 ± 11.3% for the 1 and 10 mg/kg doses of AG-0029, respectively. Conclusions: Target engagement of AG-0029 as an agonist at dopamine D2/D3 receptors and an antagonist at histamine H3 receptors could be demonstrated in the rat brain with [11C]raclopride and [11C]GSK-189254 PET, respectively. The measured occupancy values reflect the previously reported high (subnanomolar) affinity of AG-0029 to D2/D3 and moderate (submicromolar) affinity to H3 receptors.
Keywords: Parkinson’s disease; [11C]GSK-189254; [11C]raclopride; anti-Parkinson drug; dopamine D2 receptor; dual-action pharmaceutical; histamine H3 receptor; pharmacokinetic modeling.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

