Isotachophoresis: Theory and Microfluidic Applications
- PMID: 35732018
- PMCID: PMC9373989
- DOI: 10.1021/acs.chemrev.1c00640
Isotachophoresis: Theory and Microfluidic Applications
Abstract
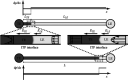
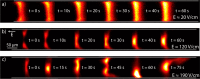
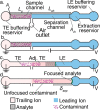
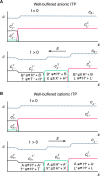
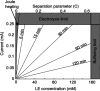
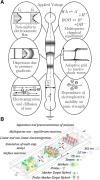
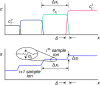
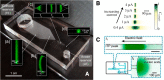
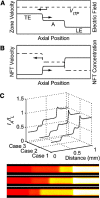
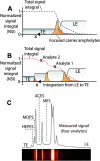
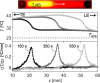
Isotachophoresis (ITP) is a versatile electrophoretic technique that can be used for sample preconcentration, separation, purification, and mixing, and to control and accelerate chemical reactions. Although the basic technique is nearly a century old and widely used, there is a persistent need for an easily approachable, succinct, and rigorous review of ITP theory and analysis. This is important because the interest and adoption of the technique has grown over the last two decades, especially with its implementation in microfluidics and integration with on-chip chemical and biochemical assays. We here provide a review of ITP theory starting from physicochemical first-principles, including conservation of species, conservation of current, approximation of charge neutrality, pH equilibrium of weak electrolytes, and so-called regulating functions that govern transport dynamics, with a strong emphasis on steady and unsteady transport. We combine these generally applicable (to all types of ITP) theoretical discussions with applications of ITP in the field of microfluidic systems, particularly on-chip biochemical analyses. Our discussion includes principles that govern the ITP focusing of weak and strong electrolytes; ITP dynamics in peak and plateau modes; a review of simulation tools, experimental tools, and detection methods; applications of ITP for on-chip separations and trace analyte manipulation; and design considerations and challenges for microfluidic ITP systems. We conclude with remarks on possible future research directions. The intent of this review is to help make ITP analysis and design principles more accessible to the scientific and engineering communities and to provide a rigorous basis for the increased adoption of ITP in microfluidics.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
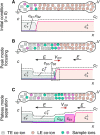
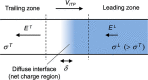
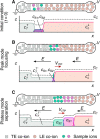
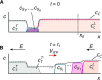



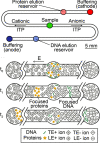
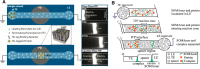

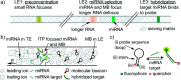

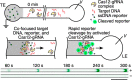
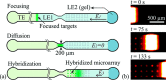


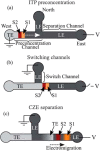
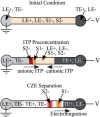
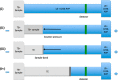

References
-
- Everaerts F. M.; Beckers J. L.; Verheggen T. P. E. M.. Isotachophoresis: Theory, Instrumentation and Applications; Elsevier, 2011.
-
- Boček P.Analytical Isotachophoresis. In Analytical Problems; Topics in Current Chemistry, Vol. 95; Springer, 1981; pp 131–177.
-
- Longsworth L. G.; MacInnes D. A. Electrophoresis of Proteins by the Tiselius Method. Chem. Rev. 1939, 24 (2), 271–287. 10.1021/cr60078a006. - DOI
-
- Tiselius A. A New Apparatus for Electrophoretic Analysis of Colloidal Mixtures. Trans. Faraday Soc. 1937, 33, 524–531. 10.1039/tf9373300524. - DOI
-
- Hall J. L. Moving Boundary Electrophoretic Study of Insulin. J. Biol. Chem. 1941, 139 (1), 175–184. 10.1016/S0021-9258(19)51374-6. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

