The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19
- PMID: 35732153
- PMCID: PMC9214726
- DOI: 10.1016/j.xcrm.2022.100663
The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19
Abstract
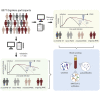
Post-acute sequelae of COVID-19 (PASC) is emerging as global problem with unknown molecular drivers. Using a digital epidemiology approach, we recruited 8,077 individuals to the cohort study for digital health research in Germany (DigiHero) to respond to a basic questionnaire followed by a PASC-focused survey and blood sampling. We report the first 318 participants, the majority thereof after mild infections. Of those, 67.8% report PASC, predominantly consisting of fatigue, dyspnea, and concentration deficit, which persists in 60% over the mean 8-month follow-up period and resolves independently of post-infection vaccination. PASC is not associated with autoantibodies, but with elevated IL-1β, IL-6, and TNF plasma levels, which we confirm in a validation cohort with 333 additional participants and a longer time from infection of 10 months. Blood profiling and single-cell data from early infection suggest the induction of these cytokines in COVID-19 lung pro-inflammatory macrophages creating a self-sustaining feedback loop.
Keywords: COVID-19; IL-1β; IL-6; PASC; SARS-CoV-2; TNF; cytokine; long covid; macrophage; post-acute sequelae of COVID-19.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
-
- Osuchowski M.F., Winkler M.S., Skirecki T., Cajander S., Shankar-Hari M., Lachmann G., Monneret G., Venet F., Bauer M., Brunkhorst F.M., et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir. Med. 2021;9:622–642. doi: 10.1016/S2213-2600(21)00218-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

