Harnessing new mHealth technologies to Strengthen the Management of Multidrug-Resistant Tuberculosis in Vietnam (V-SMART trial): a protocol for a randomised controlled trial
- PMID: 35732397
- PMCID: PMC9226862
- DOI: 10.1136/bmjopen-2021-052633
Harnessing new mHealth technologies to Strengthen the Management of Multidrug-Resistant Tuberculosis in Vietnam (V-SMART trial): a protocol for a randomised controlled trial
Abstract
Introduction: Multidrug-resistant tuberculosis (MDR-TB) remains a major public health problem globally. Long, complex treatment regimens coupled with frequent adverse events have resulted in poor treatment adherence and patient outcomes. Smartphone-based mobile health (mHealth) technologies offer national TB programmes an appealing platform to improve patient care and management; however, clinical trial evidence to support their use is lacking. This trial will test the hypothesis that an mHealth intervention can improve treatment success among patients with MDR-TB and is cost-effective compared with standard practice.
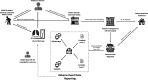
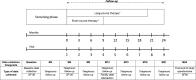
Methods and analysis: A community-based, open-label, parallel-group randomised controlled trial will be conducted among patients treated for MDR-TB in seven provinces of Vietnam. Patients commencing therapy for microbiologically confirmed rifampicin-resistant or multidrug-resistant tuberculosis within the past 30 days will be recruited to the study. Participants will be individually randomised to an intervention arm, comprising use of an mHealth application for treatment support, or a 'standard care' arm. In both arms, patients will be managed by the national TB programme according to current national treatment guidelines. The primary outcome measure of effectiveness will be the proportion of patients with treatment success (defined as treatment completion and/or bacteriological cure) after 24 months. A marginal Poisson regression model estimated via a generalised estimating equation will be used to test the effect of the intervention on treatment success. A prospective microcosting of the intervention and within-trial cost-effectiveness analysis will also be undertaken from a societal perspective. Cost-effectiveness will be presented as an incremental cost per patient successfully treated and an incremental cost per quality-adjusted life-year gained.
Ethics: Ethical approval for the study was granted by The University of Sydney Human Research Ethics Committee (2019/676).
Dissemination: Study findings will be disseminated to participants and published in peer-reviewed journals and conference proceedings.
Trial registration number: ACTRN12620000681954.
Keywords: public health; respiratory medicine (see thoracic medicine); tuberculosis.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- Ahlburg DA. Stop TB Initiative & Ministerial Conference on Tuberculosis and Sustainable Developmen: The Economic impacts of tuberculosis. World Health Organization, 2000.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
