Neuronal hyperexcitability in Alzheimer's disease: what are the drivers behind this aberrant phenotype?
- PMID: 35732622
- PMCID: PMC9217953
- DOI: 10.1038/s41398-022-02024-7
Neuronal hyperexcitability in Alzheimer's disease: what are the drivers behind this aberrant phenotype?
Abstract
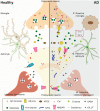
Alzheimer's disease (AD) is a progressive neurodegenerative disorder leading to loss of cognitive abilities and ultimately, death. With no cure available, limited treatments mostly focus on symptom management. Identifying early changes in the disease course may provide new therapeutic targets to halt or reverse disease progression. Clinical studies have shown that cortical and hippocampal hyperactivity are a feature shared by patients in the early stages of disease, progressing to hypoactivity during later stages of neurodegeneration. The exact mechanisms causing neuronal excitability changes are not fully characterized; however, animal and cell models have provided insights into some of the factors involved in this phenotype. In this review, we summarize the evidence for neuronal excitability changes over the course of AD onset and progression and the molecular mechanisms underpinning these differences. Specifically, we discuss contributors to aberrant neuronal excitability, including abnormal levels of intracellular Ca2+ and glutamate, pathological amyloid β (Aβ) and tau, genetic risk factors, including APOE, and impaired inhibitory interneuron and glial function. In light of recent research indicating hyperexcitability could be a predictive marker of cognitive dysfunction, we further argue that the hyperexcitability phenotype could be leveraged to improve the diagnosis and treatment of AD, and present potential targets for future AD treatment development.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Prince M, Wimo A, Guerchet M, Gemma-Claire Ali M, Wu Y-T, Prina M, et al. World Alzheimer Report 2015–The global impact of dementia: an analysis of prevalence, incidence, cost and trends. London: Alzheimer's Disease International; 2015.
-
- Alzheimer’s Association. 2019 Alzheimer’s disease facts and figures. Includes a special report on Alzheimer’s detection in the primary care setting: connecting patients and physicians. Alzheimer’s Dement J Alzheimer’s Assoc. 2019;15:321–87. doi: 10.1016/j.jalz.2019.01.010. - DOI
-
- Brion JP, Couck AM, Passareiro E, Flament-Durand J. Neurofibrillary tangles of Alzheimer’s disease: An immunohistochemical study. J Submicrosc Cytol. 1985;17:89–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

