Dissociation between individual differences in self-reported pain intensity and underlying fMRI brain activation
- PMID: 35732637
- PMCID: PMC9218124
- DOI: 10.1038/s41467-022-31039-3
Dissociation between individual differences in self-reported pain intensity and underlying fMRI brain activation
Abstract
Pain is an individual experience. Previous studies have highlighted changes in brain activation and morphology associated with within- and interindividual pain perception. In this study we sought to characterize brain mechanisms associated with between-individual differences in pain in a sample of healthy adolescent and adult participants (N = 101). Here we show that pain ratings varied widely across individuals and that individuals reported changes in pain evoked by small differences in stimulus intensity in a manner congruent with their pain sensitivity, further supporting the utility of subjective reporting as a measure of the true individual experience. Furthermore, brain activation related to interindividual differences in pain was not detected, despite clear sensitivity of the Blood Oxygenation Level-Dependent (BOLD) signal to small differences in noxious stimulus intensities within individuals. These findings suggest fMRI may not be a useful objective measure to infer reported pain intensity.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
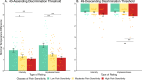



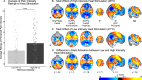

Comment in
-
Interindividual differences in pain can be explained by fMRI, sociodemographic, and psychological factors.Nat Commun. 2024 Sep 10;15(1):7883. doi: 10.1038/s41467-024-51910-9. Nat Commun. 2024. PMID: 39256362 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

