Post combustion CO2 capture with calcium and lithium hydroxide
- PMID: 35732859
- PMCID: PMC9218122
- DOI: 10.1038/s41598-022-14235-5
Post combustion CO2 capture with calcium and lithium hydroxide
Abstract
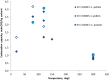
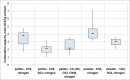
A small-scale plant was built for measuring the ability of solid sorbents towards the capture of carbon dioxide (CO2) in exhaust flue gas from an internal combustion engine. The investigated sorbents were calcium and lithium hydroxides. Both sorbents are low cost and used in the breathing gas purification systems. The carbonation capacity of each sorbent was measured for different sorbent granulometry (pellets and powder), different temperature (from ambient up to 300 °C), gas space velocity, moisture content and chemical composition of the gaseous stream. The aim was, in fact, to expose the sorbents to a gas stream with chemical and physical parameters close to those at the exhaust of an internal combustion engine. Carbonation capacity was measured with a double technique: on-line by continuously CO2 measurement with a non-dispersive infrared analyzer and off-line by using scanning electron microscopy on carbonated sorbents. Experimental results showed good CO2 uptake capacity of calcium hydroxide at low temperature (between 20 and 150 °C). Performance improvements came from the fine granulometry due to the increased exposed surface area; moreover, the presence of the moisture in gas stream also enhanced CO2 capture. The presence of sulphur dioxide and nitric oxide, instead, greatly decreased the carbonation capacity of sorbents.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








References
-
- European Environment Agency (EEA): Annual European Union greenhouse gas inventory 1990–2017 and inventory report 2019. Report n.EEA/PUBL/2019/051 https://www.eea.europa.eu/publications/european-union-greenhouse-gas-inv... (2019). Accessed 04 November 2021
-
- Straubinger A, Verhoef ET, de Groot HLF. Going electric: Environmental and welfare impacts of urban ground and air transport. Transp. Res. Part D: Transp. Environ. 2022;102:103146. doi: 10.1016/j.trd.2021.103146. - DOI
-
- Plötz P, Gnann T, Jochem P, Ümitcan Yilmaz H, Kaschub T. Impact of electric trucks powered by overhead lines on the European electricity system and CO2 emissions. Energy Policy. 2019;130:32–40. doi: 10.1016/j.enpol.2019.03.042. - DOI
-
- Oldenbroek V, Wijtzes S, Blok K, van Wijk AJM. Fuel cell electric vehicles and hydrogen balancing 100 percent renewable and integrated national transportation and energy systems. Energy Convers. Manag.: X. 2021;9:100077. doi: 10.1016/j.ecmx.2021.100077. - DOI
-
- Feenstra M, Monteiro J, van den Akker JT, Abu-Zahra MRM, Gilling E, Goetheer E. Ship-based carbon capture onboard of diesel or LNG-fuelled ships. Int. J. Greenh. Gas Control. 2019 doi: 10.1016/j.ijggc.2019.03.008. - DOI
LinkOut - more resources
Full Text Sources

