Probiotics and their beneficial effects on alcohol-induced liver injury in a rat model: the role of fecal microbiota
- PMID: 35733194
- PMCID: PMC9215017
- DOI: 10.1186/s12906-022-03643-9
Probiotics and their beneficial effects on alcohol-induced liver injury in a rat model: the role of fecal microbiota
Abstract
Background: Current therapies for alcohol-induced liver injury are of limited efficacy and associated with significant side effects. With the proposed pathophysiology of alcohol-induced liver injury to be related to deranged gut microbiota, we hypothesized that probiotics would have beneficial effects in attenuating alcohol-induced liver injury.
Methods: Twenty-four male Sprague-Dawley rats were divided into 4 groups: control group, alcohol group, Lactobacillus plantarum group, and mixed-strain probiotics group. After 4 weeks, all rats were sacrificed, and blood samples were analyzed for ALT, lipopolysaccharide level (LPS), interleukin 6 (IL-6), and tumor necrosis factor-alpha (TNF-α). Liver tissues were processed for histopathology, malondialdehyde (MDA) level and immunohistochemistry for toll-like receptors 4 (TLR-4). Stool samples were collected, and 16S rRNA sequencing was used to analyze the fecal microbiota.
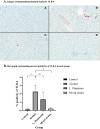
Results: Liver histopathology showed the presence of significant hepatocyte ballooning in the alcohol group as compared with the control group, and the treatment with L. plantarum or mixed-strain probiotics alleviated these changes. Significant elevation of serum ALT, LPS, IL-6, and TNF-α, hepatic MDA levels, and hepatic TLR-4 expression were observed in alcohol-fed rats as compared with control rats. The administration of L. plantarum or mixed-strain probiotics restored these changes to the levels of control rats. The relative abundance of fecal bacteria at genus level showed a significant reduction in Allobaculum, Romboutsia, Bifidobacterium, and Akkermansia in the alcohol group as compared with the control group. In probiotics-treated rats, significant increases in Allobaculum and Bifidobacterium were observed, while the relative abundance of Romboutsia and Akkermansia was unchanged compared to the alcohol group. A reduction in alpha diversity was observed in alcohol-treated rats, whereas the improvement was noted after probiotic treatment.
Conclusions: The treatment with Lactobacillus, whether as single-, or mixed-strain probiotics, was beneficial in reducing the severity of alcohol-induced liver injury likely through the increase in beneficial bacteria, and the reduction of inflammatory responses, and oxidative stress.
Keywords: Alcohol-induced liver injury; Microbiota; Probiotics; Rat model.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Single and Mixed Strains of Probiotics Reduced Hepatic Fat Accumulation and Inflammation and Altered Gut Microbiome in a Nonalcoholic Steatohepatitis Rat Model.Biomedicines. 2024 Aug 14;12(8):1847. doi: 10.3390/biomedicines12081847. Biomedicines. 2024. PMID: 39200311 Free PMC article.
-
Therapeutic Potential of Lactiplantibacillus plantarum FB091 in Alleviating Alcohol-Induced Liver Disease through Gut-Liver Axis.J Microbiol Biotechnol. 2024 Oct 28;34(10):2100-2111. doi: 10.4014/jmb.2407.07051. Epub 2024 Aug 9. J Microbiol Biotechnol. 2024. PMID: 39300956 Free PMC article.
-
[Probiotics enhance the efficacy of fecal microbiota transplantation in severe acute liver injury].Zhonghua Gan Zang Bing Za Zhi. 2020 Apr 20;28(4):345-350. doi: 10.3760/cma.j.cn501113-20190823-00315. Zhonghua Gan Zang Bing Za Zhi. 2020. PMID: 32403888 Chinese.
-
Gut Microbiota as Emerging Players in the Development of Alcohol-Related Liver Disease.Biomedicines. 2024 Dec 31;13(1):74. doi: 10.3390/biomedicines13010074. Biomedicines. 2024. PMID: 39857657 Free PMC article. Review.
-
Harnessing the potential of probiotics in the treatment of alcoholic liver disorders.Front Pharmacol. 2023 Jun 9;14:1212742. doi: 10.3389/fphar.2023.1212742. eCollection 2023. Front Pharmacol. 2023. PMID: 37361234 Free PMC article. Review.
Cited by
-
Comparing Transgenic Production to Supplementation of ω-3 PUFA Reveals Distinct But Overlapping Mechanisms Underlying Protection Against Metabolic and Hepatic Disorders.Function (Oxf). 2022 Dec 29;4(2):zqac069. doi: 10.1093/function/zqac069. eCollection 2023. Function (Oxf). 2022. PMID: 36778746 Free PMC article.
-
Single and Mixed Strains of Probiotics Reduced Hepatic Fat Accumulation and Inflammation and Altered Gut Microbiome in a Nonalcoholic Steatohepatitis Rat Model.Biomedicines. 2024 Aug 14;12(8):1847. doi: 10.3390/biomedicines12081847. Biomedicines. 2024. PMID: 39200311 Free PMC article.
-
Therapeutic Potential of Lactiplantibacillus plantarum FB091 in Alleviating Alcohol-Induced Liver Disease through Gut-Liver Axis.J Microbiol Biotechnol. 2024 Oct 28;34(10):2100-2111. doi: 10.4014/jmb.2407.07051. Epub 2024 Aug 9. J Microbiol Biotechnol. 2024. PMID: 39300956 Free PMC article.
-
Impact of high-fructose diet and metformin on histomorphological and molecular parameters of reproductive organs and vaginal microbiota of female rat.Sci Rep. 2024 Nov 10;14(1):27463. doi: 10.1038/s41598-024-76211-5. Sci Rep. 2024. PMID: 39523383 Free PMC article.
-
Levilactobacillus brevis MG5311 Alleviates Ethanol-Induced Liver Injury by Suppressing Hepatic Oxidative Stress in C57BL/6 Mice.Microorganisms. 2022 Dec 15;10(12):2488. doi: 10.3390/microorganisms10122488. Microorganisms. 2022. PMID: 36557739 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

