Probing local chromatin dynamics by tracking telomeres
- PMID: 35733342
- PMCID: PMC9382323
- DOI: 10.1016/j.bpj.2022.06.020
Probing local chromatin dynamics by tracking telomeres
Abstract
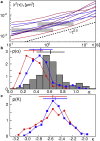
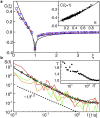
Chromatin dynamics is key for cell viability and replication. In interphase, chromatin is decondensed, allowing the transcription machinery to access a plethora of DNA loci. Yet, decondensed chromatin occupies almost the entire nucleus, suggesting that DNA molecules can hardly move. Recent reports have even indicated that interphase chromatin behaves like a solid body on mesoscopic scales. To explore the local chromatin dynamics, we have performed single-particle tracking on telomeres under varying conditions. We find that mobile telomeres feature, under all conditions, a strongly subdiffusive, antipersistent motion that is consistent with the monomer motion of a Rouse polymer in viscoelastic media. In addition, telomere trajectories show intermittent accumulations in local niches at physiological conditions, suggesting that the surrounding chromatin reorganizes on these timescales. Reducing the temperature or exposing cells to osmotic stress resulted in a significant reduction of mobile telomeres and the number of visited niches. Altogether, our data indicate a vivid local chromatin dynamics, akin to a semidilute polymer solution, unless perturbations enforce a more rigid or entangled state of chromatin.
Copyright © 2022 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Alberts B. Sixth edition. Garland Science, Taylor and Francis Group; 2015. Molecular Biology of the Cell; p. 1.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

