Outcomes of Patients with EGFR- Mutant Advanced NSCLC in a Developing Country in Southeast Asia
- PMID: 35733510
- PMCID: PMC9208817
- DOI: 10.2147/CMAR.S364713
Outcomes of Patients with EGFR- Mutant Advanced NSCLC in a Developing Country in Southeast Asia
Abstract
Background: Although first- and second-generation EGFR TKIs are considered first-line treatment in EGFRm+ NSCLC, most patients develop resistance and progress, commonly, EGFR T790M mutation. The third-generation EGFR-TKI has demonstrated efficacy in patients with progressive disease harboring the T790M mutation and in the first-line setting, bypassing this mode of resistance. The primary objectives of this study are to describe the proportion of EGFRm+ NSCLC patients treated with first-, second- and third-generation EGFR TKIs, and cytotoxic chemotherapy in the first-line setting, and the time on treatment for each category. Secondary objectives are to determine the dropout rate, the rates for T790M mutation testing at disease progression and the type of subsequent treatment.
Methods: This multicenter retrospective study utilized data from the Malaysian Lung Cancer Registry that actively registers all lung cancer patients ≥18 years, with primary lung cancer confirmed histologically or cytologically. All patients diagnosed with advanced stages (ie stages IIIB, IIIC and IV) EGFRm+ NSCLC from 1st of January 2015 to 31st December 2019 were included.
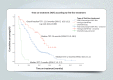
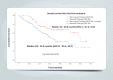
Results: Of 406 patients with EGFRm+ NCSLC, 351 were treated. Types of first-line treatment were as follows: EGFR-TKIs (first generation - 54.1%, second generation - 25.6% and third-generation - 12.5%) and chemotherapy (7.7%). The median time of treatment for each generation of EGFR-TKI was 12 months, 12 months and 24 months, and 2 months for chemotherapy. The dropout rate was 28.7% (n = 101). Nearly half (49.4%) of patients who were on first- or second-generation EGFR-TKI had further genetic testing via liquid or tissue biopsies upon disease progression. About 24.9% of those who developed disease progression after first- or second-generation EGFR TKI were started on a third-generation EGFR TKI.
Conclusion: In the real-world, the management of EGFRm+ advanced NSCLC patients in an Asian cost-restrictive setting may adversely affect the choice of first-line therapy, time on each line of treatment and subsequently the overall survival of patients.
Keywords: lung cancer; overall survival; time on treatment; tyrosine kinase inhibitors.
© 2022 How et al.
Conflict of interest statement
CKL has received research grants and honoraria from Astra Zeneca, Boehringer Ingelheim, MSD, Novartis, Pfizer, Roche and Zuellig Pharma. HHH has received research grants and honoraria from Astra Zeneca, EISAI, MSD, Novartis and Tessa Therapeutics; AB science, AdipoLab, Arcus Bioscience. GFH has received research grants and honoraria from AB Science, Arcus Biosciences, Ipsen, Astellas, Astra Zeneca, Eli Lily, Boehringer Ingelheim, BMS, MSD, MIRARI Therapeutics, Novartis, Pfizer, Regeneron, Roche and Tessa Therapeutics, respectively. LMT has received honoraria from Astra Zeneca, Boehringer Ingelheim, Roche, MSD and Pfizer. The other authors have no conflicts of interest to declare in this work.
Figures


References
-
- Ministry of Health, Malaysia. Malaysia National Cancer Registry report 2012–2016; 2019.
-
- Ministry of Health, Malaysia. Malaysian study on cancer survival (MySCan); 2018.
-
- Kan CS, Chan KM. A review of lung cancer research in Malaysia. Med J Malaysia. 2016;71(Suppl 1):70–78. - PubMed
-
- Werutsky G, Debiasi M, Sampaio FH, et al. P1.08: updated analysis of global epidemiology of EGFR Mutation in advanced non-small cell lung cancer: track: prevention, early detection, epidemiology and tobacco control. J Thorac Oncol. 2016;11(10):S184–S185. doi:10.1016/j.jtho.2016.08.030 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

