Generation of immunocompetent syngeneic allograft mouse models for pediatric diffuse midline glioma
- PMID: 35733514
- PMCID: PMC9210310
- DOI: 10.1093/noajnl/vdac079
Generation of immunocompetent syngeneic allograft mouse models for pediatric diffuse midline glioma
Erratum in
-
Corrigendum to: Generation of immunocompetent syngeneic allograft mouse models for pediatric diffuse midline glioma.Neurooncol Adv. 2023 Jun 10;5(1):vdad072. doi: 10.1093/noajnl/vdad072. eCollection 2023 Jan-Dec. Neurooncol Adv. 2023. PMID: 37303863 Free PMC article.
Abstract
Background: Diffuse midline gliomas (DMG) are highly malignant incurable pediatric brain tumors. A lack of effective treatment options highlights the need to investigate novel therapeutic strategies. This includes the use of immunotherapy, which has shown promise in other hard-to-treat tumors. To facilitate preclinical immunotherapeutic research, immunocompetent mouse models that accurately reflect the unique genetic, anatomical, and histological features of DMG patients are warranted.
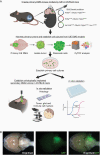
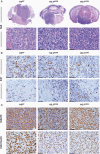
Methods: We established cell cultures from primary DMG mouse models (C57BL/6) that were generated by brainstem targeted intra-uterine electroporation (IUE). We subsequently created allograft DMG mouse models by orthotopically implanting these tumor cells into syngeneic mice. Immunohistochemistry and -fluorescence, mass cytometry, and cell-viability assays were then used to verify that these murine tumors recapitulated human DMG.
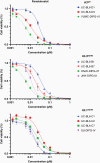
Results: We generated three genetically distinct allograft models representing histone 3 wildtype (H3WT) and K27M-mutant DMG (H3.3K27M and H3.1K27M). These allograft models recapitulated the histopathologic phenotype of their human counterparts, including their diffuse infiltrative growth and expression of DMG-associated antigens. These murine pontine tumors also exhibited an immune microenvironment similar to human DMG, characterized by considerable myeloid cell infiltration and a paucity of T-lymphocytes and NK cells. Finally, we show that these murine DMG cells display similar sensitivity to histone deacetylase (HDAC) inhibition as patient-derived DMG cells.
Conclusions: We created and validated an accessible method to generate immunocompetent allograft models reflecting different subtypes of DMG. These models adequately recapitulated the histopathology, immune microenvironment, and therapeutic response of human DMG, providing useful tools for future preclinical studies.
Keywords: DIPG; DMG; immunotherapy | syngeneic allograft; tumor microenvironment.
© The Author(s) 2022. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Figures


References
-
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. - PubMed
-
- Sturm D, Pfister SM, Jones DTW. Pediatric gliomas: current concepts on diagnosis, biology, and clinical management. J Clin Oncol. 2017;35(21):2370–2377. - PubMed
-
- Meel MH, Kaspers GJL, Hulleman E. Preclinical therapeutic targets in diffuse midline glioma. Drug Resist Updat. 2019;44:15–25. - PubMed
