Promising Health Benefits of Adjuvant Acmella and Zingiber Extracts Combined with Coenzyme Q10 Phytosomes, Supplementation in Chronic Pain Treated with Medical Cannabis: A Prospective and Open-Label Clinical Study
- PMID: 35733629
- PMCID: PMC9208939
- DOI: 10.1155/2022/7099161
Promising Health Benefits of Adjuvant Acmella and Zingiber Extracts Combined with Coenzyme Q10 Phytosomes, Supplementation in Chronic Pain Treated with Medical Cannabis: A Prospective and Open-Label Clinical Study
Abstract
Background: Chronic pain is a condition where pain persists for months or even years. Nowadays, several drugs comprising of medical cannabis are utilized for chronic pain relief. Even if there are some associated side effects, the use of supplements can widen the reliable tools available for improving an individual's quality of life.
Objective: The aim of the present study was to evaluate the efficacy in terms of pain intensity, psychological well-being, and quality of life of a new dietary supplement in chronic pain subjects under current treatment with medical cannabis.
Methods: In this pilot study, 48 medical cannabis-treated subjects were supplemented with a dietary supplement containing a combination of standardized Zingiber officinalis and Acmella oleracea extracts in phytosome (Mitidol), coenzyme Q10 phytosome (Ubiqsome), and group B vitamins (B1, B6, and B12), twice daily for 90 days. In order to explore the benefits of the product as an adjuvant supplementation for pain relief, the pain intensity, measured by the visual analogue scale (VAS), the pain type, and quality, evaluated by the Italian Pain Questionnaire (QUID) and the possible reduction of therapeutic and/or painkiller doses were recorded.
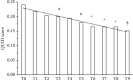
Results: After 90 days, significant pain relief was detected in almost 70% of the subjects receiving the new dietary supplement, with sensory, emotional, and pain amelioration in one-third of them. A reduction in both tetrahydrocannabinol (THC) and cannabidiol (CBD) doses was also observed after 3 months of supplementation. These findings demonstrate new perspectives for the use of an innovative dietary supplement that combines Acmella and Zingiber extracts, Coenzyme Q10, and group B vitamins resulting in a beneficial long-term adjuvant in cannabis-treated pain subjects.
Copyright © 2022 Paolo Poli et al.
Conflict of interest statement
AR, GP, and MM are employees of Indena SpA. PLD and SB are employees of Farmad Lab, Firenze. The other authors declare that they have no conflicts of interest.
Figures


References
-
- Health. DM 9/11/2015 ministerial decree. 2015. https://www.gazzettaufficiale.it/eli/id/2015/11/30/15A08888/sg .
LinkOut - more resources
Full Text Sources

