Study and application of graphene oxide in the synthesis of 2,3-disubstituted quinolines via a Povarov multicomponent reaction and subsequent oxidation
- PMID: 35733657
- PMCID: PMC9135005
- DOI: 10.1039/d2ra01752k
Study and application of graphene oxide in the synthesis of 2,3-disubstituted quinolines via a Povarov multicomponent reaction and subsequent oxidation
Abstract
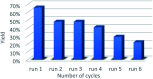
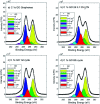
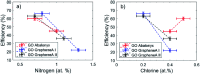
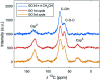
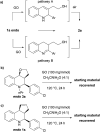
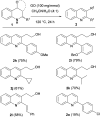
The carbocatalyzed synthesis of 2,3-disubstituted quinolines is disclosed. This process involved a three-component Povarov reaction of anilines, aldehydes and electron-enriched enol ethers, which gave the substrate for the subsequent oxidation. Graphene oxide (GO) was exploited as a heterogeneous, metal-free and sustainable catalyst for both transformations. The multicomponent reaction proceeded under simple and mild reaction conditions, exhibited good functional group tolerance, and could be easily scaled up to the gram level. A selection of tetrahydroquinolines obtained was subsequently aromatized to quinolines. The multistep synthesis could also be performed as a one-pot procedure. Investigation of the real active sites of GO was carried out by performing control experiments and a by full characterization of the carbon material by X-ray photoelectron spectroscopy (XPS) and solid-state nuclear magnetic resonance (ssNMR).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





Similar articles
-
Diastereoselective, multicomponent access to trans-2-aryl-4-arylamino-1,2,3,4-tetrahydroquinolines via an AA'BC sequential four-component reaction and their application to 2-arylquinoline synthesis.Org Biomol Chem. 2013 Jan 28;11(4):569-79. doi: 10.1039/c2ob26754c. Epub 2012 Oct 22. Org Biomol Chem. 2013. PMID: 23090014
-
Chiral phosphoric acid-catalyzed enantioselective three-component Povarov reaction using enecarbamates as dienophiles: highly diastereo- and enantioselective synthesis of substituted 4-aminotetrahydroquinolines.J Am Chem Soc. 2011 Sep 21;133(37):14804-13. doi: 10.1021/ja205891m. Epub 2011 Aug 26. J Am Chem Soc. 2011. PMID: 21827206
-
Regioselective three-component synthesis of 2,3-disubstituted quinolines via the enaminone modified Povarov reaction.Org Biomol Chem. 2017 Nov 22;15(45):9585-9589. doi: 10.1039/c7ob02411h. Org Biomol Chem. 2017. PMID: 29114679
-
Tetrahydroquinolines by the multicomponent Povarov reaction in water: calix[n]arene-catalysed cascade process and mechanistic insights.Org Biomol Chem. 2019 Mar 13;17(11):2913-2922. doi: 10.1039/c8ob02928h. Org Biomol Chem. 2019. PMID: 30724962
-
Bio-reduction of Graphene Oxide: Catalytic Applications of (Reduced) GO in Organic Synthesis.Curr Org Synth. 2020;17(3):164-191. doi: 10.2174/1570179417666200115110403. Curr Org Synth. 2020. PMID: 32538718 Review.
Cited by
-
Synthesis of symmetric bis-α-ketoamides from renewable starting materials and comparative study of their nucleating efficiency in PLLA.RSC Adv. 2023 Feb 8;13(8):4994-5001. doi: 10.1039/d2ra07934h. eCollection 2023 Feb 6. RSC Adv. 2023. PMID: 36762081 Free PMC article.
-
Synthesis of spiroindolenines through a one-pot multistep process mediated by visible light.Beilstein J Org Chem. 2024 Oct 29;20:2722-2731. doi: 10.3762/bjoc.20.230. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 39498445 Free PMC article.
-
Fabrication of a novel graphene oxide based magnetic nanocomposite and its usage as a highly effectual catalyst for the construction of N,N'-alkylidene bisamides.RSC Adv. 2024 Aug 13;14(35):25235-25246. doi: 10.1039/d4ra04136d. eCollection 2024 Aug 12. RSC Adv. 2024. PMID: 39139253 Free PMC article.
-
Functionalized graphene oxide by 4-amino-3-hydroxy-1-naphthalenesulfonic acid as a heterogeneous nanocatalyst for the one-pot synthesis of tetraketone and tetrahydrobenzo[b]pyran derivatives under green conditions.Nanoscale Adv. 2024 May 24;6(15):3911-3922. doi: 10.1039/d4na00223g. eCollection 2024 Jul 23. Nanoscale Adv. 2024. PMID: 39050950 Free PMC article.
References
-
- Anastas P. T. Zimmerman J. B. The United Nations sustainability goals: How can sustainable chemistry contribute? Curr. Opin. Green Sustain. 2018;13:150–153.
-
- Axon S. James D. The UN Sustainable Development Goals: How can sustainable chemistry contribute? A view from the chemical industry. Curr. Opin. Green Sustain. 2018;13:140–145.
-
- Jiang L. and Yi W., in Sustainable Organic Synthesis: Tools and Strategies, The Royal Society of Chemistry, 2022, 10.1039/9781839164842-00472, pp. 472–487 - DOI
-
- Green N. J. Sherburn M. S. Multi-Bond Forming Processes in Efficient Synthesis. Aust. J. Chem. 2013;66:267.
LinkOut - more resources
Full Text Sources

