Study and application of graphene oxide in the synthesis of 2,3-disubstituted quinolines via a Povarov multicomponent reaction and subsequent oxidation
- PMID: 35733657
- PMCID: PMC9135005
- DOI: 10.1039/d2ra01752k
Study and application of graphene oxide in the synthesis of 2,3-disubstituted quinolines via a Povarov multicomponent reaction and subsequent oxidation
Abstract
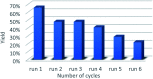
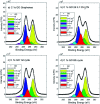
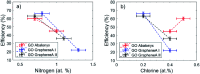
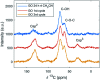
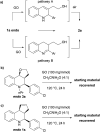
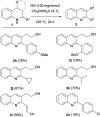
The carbocatalyzed synthesis of 2,3-disubstituted quinolines is disclosed. This process involved a three-component Povarov reaction of anilines, aldehydes and electron-enriched enol ethers, which gave the substrate for the subsequent oxidation. Graphene oxide (GO) was exploited as a heterogeneous, metal-free and sustainable catalyst for both transformations. The multicomponent reaction proceeded under simple and mild reaction conditions, exhibited good functional group tolerance, and could be easily scaled up to the gram level. A selection of tetrahydroquinolines obtained was subsequently aromatized to quinolines. The multistep synthesis could also be performed as a one-pot procedure. Investigation of the real active sites of GO was carried out by performing control experiments and a by full characterization of the carbon material by X-ray photoelectron spectroscopy (XPS) and solid-state nuclear magnetic resonance (ssNMR).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





References
-
- Anastas P. T. Zimmerman J. B. The United Nations sustainability goals: How can sustainable chemistry contribute? Curr. Opin. Green Sustain. 2018;13:150–153.
-
- Axon S. James D. The UN Sustainable Development Goals: How can sustainable chemistry contribute? A view from the chemical industry. Curr. Opin. Green Sustain. 2018;13:140–145.
-
- Jiang L. and Yi W., in Sustainable Organic Synthesis: Tools and Strategies, The Royal Society of Chemistry, 2022, 10.1039/9781839164842-00472, pp. 472–487 - DOI
-
- Green N. J. Sherburn M. S. Multi-Bond Forming Processes in Efficient Synthesis. Aust. J. Chem. 2013;66:267.
LinkOut - more resources
Full Text Sources

