Characterization of the Interrenal Gland and Sexual Traits Development in cyp17a2-Deficient Zebrafish
- PMID: 35733778
- PMCID: PMC9207535
- DOI: 10.3389/fendo.2022.910639
Characterization of the Interrenal Gland and Sexual Traits Development in cyp17a2-Deficient Zebrafish
Abstract
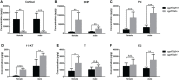
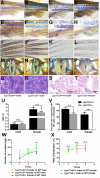
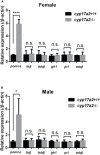
Unlike the Cytochrome P450, family 17, subfamily A, member 1 (Cyp17a1), which possesses both 17α-hydroxylase and 17,20-lyase activities involved in the steroidogenic pathway that produces androgens and estrogens, Cytochrome P450, family 17, subfamily A, polypeptide 2 (Cyp17a2) possesses only 17α-hydroxylase activity and is known essential for the synthesis of cortisol. Besides with expressed in testes and ovaries, where the cyp17a1 is mainly expressed, cyp17a2 is also expressed in the interrenal gland in fish. Until now, the roles of cyp17a2 in fish, especially in sexual traits development and hypothalamic-pituitary-interrenal (HPI) axis, are poorly studied. To investigate the roles of Cyp17a2 in teleosts, the cyp17a2-null zebrafish was generated and analyzed by us. The significantly decreased cortisol concentration was observed both in the cyp17a2-deficient males and females at adult stage. The interrenal gland enlargement, increased pituitary proopiomelanocortin a (pomca) expression, decreased locomotion activity and response to light-stimulated stress were observed in cyp17a2-deficient fish. Intriguingly, the cyp17a2-deficient males were fertile and with normal breeding tubercles on the pectoral fin, but females were infertile, deficient in genital papilla and with decreased gonadosomatic index (GSI). The increased progesterone (P4), 17α,20β-dihydroxy-4-pregnen-3-one (DHP) and 11-ketotestosterone (11-KT) in the cyp17a2-deficient males and females were observed. The increased concentration of testosterone (T) and estradiol (E2) was observed in cyp17a2-/- females and cyp17a2-/- males, respectively. By examining the ovaries development of cyp17a2-deficient fish at 3 months postfertilization (mpf), we observed that the oocytes were over-activated. Taken together, our findings demonstrate that Cyp17a2 is indispensable for production and physiology of cortisol, and cyp17a2-deficiency resulted in diminished cortisol but accumulated P4 and DHP, which may result in the over-activated oocytes in cyp17a2-deficient females.
Keywords: cortisol; cyp17a2; oocyte maturation; sexual trait; zebrafish.
Copyright © 2022 Shi, Shu, Li, Lou, Jin, He, Yin and Zhai.
Conflict of interest statement
Author TS was employed by China Three Gorges Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Cyp17a2 is involved in testicular development and fertility in male Nile tilapia, Oreochromis niloticus.Front Endocrinol (Lausanne). 2022 Nov 29;13:1074921. doi: 10.3389/fendo.2022.1074921. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36523590 Free PMC article.
-
Characterization and expression of cDNAs encoding P450c17-II (cyp17a2) in Japanese eel during induced ovarian development.Gen Comp Endocrinol. 2015 Sep 15;221:134-43. doi: 10.1016/j.ygcen.2015.01.026. Epub 2015 Feb 18. Gen Comp Endocrinol. 2015. PMID: 25701739
-
Characterization of Sexual Trait Development in cyp17a1-Deficient Zebrafish.Endocrinology. 2018 Oct 1;159(10):3549-3562. doi: 10.1210/en.2018-00551. Endocrinology. 2018. PMID: 30202919
-
Interrenal development and function in zebrafish.Mol Cell Endocrinol. 2021 Sep 15;535:111372. doi: 10.1016/j.mce.2021.111372. Epub 2021 Jun 24. Mol Cell Endocrinol. 2021. PMID: 34175410 Review.
-
Molecular programming of the corticosteroid stress axis during zebrafish development.Comp Biochem Physiol A Mol Integr Physiol. 2009 May;153(1):49-54. doi: 10.1016/j.cbpa.2008.12.008. Epub 2008 Dec 25. Comp Biochem Physiol A Mol Integr Physiol. 2009. PMID: 19146973 Review.
Cited by
-
Cyp17a2 is involved in testicular development and fertility in male Nile tilapia, Oreochromis niloticus.Front Endocrinol (Lausanne). 2022 Nov 29;13:1074921. doi: 10.3389/fendo.2022.1074921. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36523590 Free PMC article.
-
Deficiency of leap2 promotes somatic growth in zebrafish: Involvement of the growth hormone system.Heliyon. 2024 Aug 15;10(18):e36397. doi: 10.1016/j.heliyon.2024.e36397. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39347412 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

