Multi-Hit White Matter Injury-Induced Cerebral Palsy Model Established by Perinatal Lipopolysaccharide Injection
- PMID: 35733809
- PMCID: PMC9207278
- DOI: 10.3389/fped.2022.867410
Multi-Hit White Matter Injury-Induced Cerebral Palsy Model Established by Perinatal Lipopolysaccharide Injection
Abstract
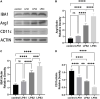
Cerebral palsy (CP) is a group of permanent, but not unchanging, disorders of movement and/or posture and motor function. Since the major brain injury associated with CP is white matter injury (WMI), especially, in preterm infants, we established a "multi-hit" rat model to mimic human WMI in symptomatology and at a histological level. In our WMI model, pups suffering from limb paresis, incoordination, and direction difficulties fit the performance of CP. Histologically, they present with fewer neural cells, inordinate fibers, and more inflammatory cell infiltration, compared to the control group. From the electron microscopy results, we spotted neuronal apoptosis, glial activation, and myelination delay. Besides, the abundant appearance of IBA1-labeled microglia also implied that microglia play a role during neuronal cell injury. After activation, microglia shift between the pro-inflammatory M1 type and the anti-inflammatory M2 type. The results showed that LPS/infection stimulated IBA1 + (marked activated microglia) expression, downregulated CD11c + (marked M1 phenotype), and upregulated Arg 1 + (marked M2 phenotype) protein expression. It indicated an M1 to M2 transition after multiple infections. In summary, we established a "multi-hit" WMI-induced CP rat model and demonstrated that the microglial activation correlates tightly with CP formation, which may become a potential target for future studies.
Keywords: animal model; cerebral palsy; electron microscopy; perinatal infection; white matter injury.
Copyright © 2022 Liu, Fang, Duan, Wang, Cui, Yang and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Modulatory effect of IL-1 inhibition following lipopolysaccharide-induced neuroinflammation in neonatal microglia and astrocytes.Int J Dev Neurosci. 2022 May;82(3):243-260. doi: 10.1002/jdn.10179. Epub 2022 Mar 27. Int J Dev Neurosci. 2022. PMID: 35315121
-
Activated protein C reduces endotoxin-induced white matter injury in the developing rat brain.Brain Res. 2007 Aug 20;1164:14-23. doi: 10.1016/j.brainres.2007.04.083. Epub 2007 May 23. Brain Res. 2007. PMID: 17644074
-
Inhibition of P2X4R attenuates white matter injury in mice after intracerebral hemorrhage by regulating microglial phenotypes.J Neuroinflammation. 2021 Aug 23;18(1):184. doi: 10.1186/s12974-021-02239-3. J Neuroinflammation. 2021. PMID: 34425835 Free PMC article.
-
White matter injury in the neonatal hypoxic-ischemic brain and potential therapies targeting microglia.J Neurosci Res. 2021 Apr;99(4):991-1008. doi: 10.1002/jnr.24761. Epub 2021 Jan 8. J Neurosci Res. 2021. PMID: 33416205 Review.
-
Impaired oligodendrocyte maturation in preterm infants: Potential therapeutic targets.Prog Neurobiol. 2016 Jan;136:28-49. doi: 10.1016/j.pneurobio.2015.11.002. Epub 2015 Dec 2. Prog Neurobiol. 2016. PMID: 26655283 Review.
Cited by
-
Oral ellagic acid attenuated LPS-induced neuroinflammation in rat brain: MEK1 interaction and M2 microglial polarization.Exp Biol Med (Maywood). 2023 Apr;248(7):656-664. doi: 10.1177/15353702231182230. Epub 2023 Jun 20. Exp Biol Med (Maywood). 2023. PMID: 37340785 Free PMC article.
-
Prenatal inflammation exacerbates hyperoxia-induced neonatal brain injury.J Neuroinflammation. 2025 Feb 28;22(1):57. doi: 10.1186/s12974-025-03389-4. J Neuroinflammation. 2025. PMID: 40022130 Free PMC article.
-
Wnt1 oversees microglial activation by the Wnt/LRP5/6 receptor signaling pathway during lipopolysaccharide-mediated toxicity.Mol Biol Rep. 2025 Mar 1;52(1):273. doi: 10.1007/s11033-025-10360-2. Mol Biol Rep. 2025. PMID: 40025242 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

