Causes of Kidney Graft Failure in a Cohort of Recipients With a Very Long-Time Follow-Up After Transplantation
- PMID: 35733857
- PMCID: PMC9207199
- DOI: 10.3389/fmed.2022.842419
Causes of Kidney Graft Failure in a Cohort of Recipients With a Very Long-Time Follow-Up After Transplantation
Abstract
Background: Biopsy-proven causes of graft loss many years after kidney transplantation are scarcely documented.
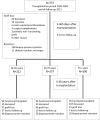
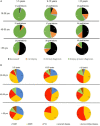
Methods: Patients transplanted between 1995 and 2005 (n = 737) in a single center were followed on a regular basis until 2021. The recipients were divided according to age at transplantation into 3 groups; 18-39 years (young), 40-55 years (middle age), and older than 55 years (elderly). For cause biopsies of renal transplants were clustered into the categories, rejection, IFTA, return original disease, and diagnosis of de novo kidney disease.
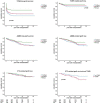
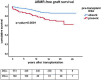
Results: Rejection was the main cause of graft failure censored for death at every time period after transplantation. The incidence of T cell-mediated rejection (TCMR) became rare 6 years after transplantation while the cumulative incidence of antibody-mediated rejection (ABMR) increased over time (1.1% per year). ABMR was not diagnosed anymore beyond 15 years of follow-up in recipients without pre-transplant donor-specific antibodies (DSA). An episode of TCMR was associated with an increased incidence of ABMR diagnosis in the short-term but did not increase the overall incidence of AMBR not in the long-term. Death as a cause of graft failure was an important competitive risk factor long after transplantation and resulted in a significantly lower frequency of rejection-related graft loss in the elderly group (11 vs. 23% in the young group at 15 year follow-up).
Conclusion: Rejection is a major cause of graft loss but recipient's age, time after transplantation, and the presence of DSA before transplantation determine the relative contribution to overall graft loss and the type of rejection involved.
Keywords: ABMR; TCMR; antibody-mediated rejection; graft failure risk; kidney transplantation; long term.
Copyright © 2022 Betjes, Roelen, van Agteren and Kal-van Gestel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Najafian B, Kasiske BL. Chronic allograft nephropathy. Curr Opin Nephrol Hypertens. (2008) 17:149–55. - PubMed
-
- Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. (2003) 349:2326–33. - PubMed
-
- El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, et al. Identifying specific causes of kidney allograft loss. Am J Transplant. (2009) 9:527–35. - PubMed
LinkOut - more resources
Full Text Sources

