Soil Microbial Communities Affect the Growth and Secondary Metabolite Accumulation in Bletilla striata (Thunb.) Rchb. f
- PMID: 35733964
- PMCID: PMC9207479
- DOI: 10.3389/fmicb.2022.916418
Soil Microbial Communities Affect the Growth and Secondary Metabolite Accumulation in Bletilla striata (Thunb.) Rchb. f
Abstract
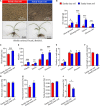
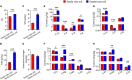
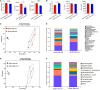
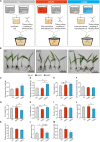
Bletilla striata (Thunb.) Rchb.f. is a perennial herb belonging to the Orchidaceae family. Its tubers are used in traditional Chinese medicine to treat gastric ulcers, inflammation, silicosis tuberculosis, and pneumogastric hemorrhage. It has been reported that different soil types can affect the growth of B. striata and the accumulation of secondary metabolites in its tubers, but the biological mechanisms underlying these effects remain unclear. In this study, we compared agronomic traits and the accumulation of secondary metabolites (extractum, polysaccharide, total phenol, militarine) in B. striata grown in sandy loam or sandy clay soil. In addition, we compared physicochemical properties and microbial communities between the two soil types. In pot experiments, we tested how irradiating soil or transplanting microbiota from clay or loam into soil affected B. striata growth and accumulation of secondary metabolites. The results showed that sandy loam and sandy clay soils differed significantly in their physicochemical properties as well as in the structure and composition of their microbial communities. Sandy loam soil had higher pH, SOM, SOC, T-Ca, T-N, T-Mg, T-Mn, T-Zn, A-Ca, A-Mn, and A-Cu than sandy clay soil, but significantly lower T-P, T-K, T-Fe, and A-P content. Sandy loam soil showed 7.32% less bacterial diversity based on the Shannon index, 19.59% less based on the Ace index, and 24.55% less based on the Chao index. The first two components of the PCoA explained 74.43% of the variation in the bacterial community (PC1 = 64.92%, PC2 = 9.51%). Similarly, the first two components of the PCoA explained 58.48% of the variation in the fungal community (PC1 = 43.67%, PC2 = 14.81%). The microbiome associated with sandy clay soil can promote the accumulation of militarine in B. striata tubers, but it inhibits the growth of B. striata. The accumulation of secondary metabolites such as militarine in B. striata was significantly higher in sandy clay than in sandy loam soil. Conversely, B. striata grew better in sandy loam soil. The microbiome associated with sandy loam soil can promote the growth of B. striata, but it reduces the accumulation of militarine in B. striata tubers. Pot experiment results further confirmed that the accumulation of secondary metabolites such as militarine was higher in soil transplanted with loam microbiota than in soil transplanted with clay microbiota. These results may help guide efforts to improve B. striata yield and its accumulation of specific secondary metabolites.
Keywords: Bletilla striata; sandy clay soil; sandy loam soil; secondary metabolites; soil microbiome.
Copyright © 2022 Xiao, Xu, Zhang, Jiang, Zhang, Yang, Xu, Zhang and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Soil microorganism colonization influenced the growth and secondary metabolite accumulation of Bletilla striata (Thunb.) Rchb. F.BMC Microbiol. 2025 May 8;25(1):276. doi: 10.1186/s12866-025-03960-2. BMC Microbiol. 2025. PMID: 40335962 Free PMC article.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Single-incision sling operations for urinary incontinence in women.Cochrane Database Syst Rev. 2017 Jul 26;7(7):CD008709. doi: 10.1002/14651858.CD008709.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2023 Oct 27;10:CD008709. doi: 10.1002/14651858.CD008709.pub4. PMID: 28746980 Free PMC article. Updated.
Cited by
-
Biosynthetic Mechanisms of Plant Chlorogenic Acid from a Microbiological Perspective.Microorganisms. 2025 May 13;13(5):1114. doi: 10.3390/microorganisms13051114. Microorganisms. 2025. PMID: 40431287 Free PMC article. Review.
-
Transcriptome Analysis of the CML Gene Family in Bletilla striata and Regulation of Militarine Synthesis Under Sodium Acetate and Salicylic Acid Treatments.Plants (Basel). 2025 Mar 28;14(7):1052. doi: 10.3390/plants14071052. Plants (Basel). 2025. PMID: 40219120 Free PMC article.
-
Advances in militarine: Pharmacology, synthesis, molecular regulation and regulatory mechanisms.Heliyon. 2024 Jan 10;10(2):e24341. doi: 10.1016/j.heliyon.2024.e24341. eCollection 2024 Jan 30. Heliyon. 2024. PMID: 38293334 Free PMC article. Review.
-
Metagenomic evidence for antibiotic-associated actinomycetes in the Karamay Gobi region.Front Microbiol. 2024 Mar 5;15:1330880. doi: 10.3389/fmicb.2024.1330880. eCollection 2024. Front Microbiol. 2024. PMID: 38505550 Free PMC article.
-
The appearance, active components, antioxidant activities, and their markers in fibrous roots of Bletilla striata.PLoS One. 2024 Nov 11;19(11):e0313318. doi: 10.1371/journal.pone.0313318. eCollection 2024. PLoS One. 2024. PMID: 39527603 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

