Cinaciguat (BAY-582667) Modifies Cardiopulmonary and Systemic Circulation in Chronically Hypoxic and Pulmonary Hypertensive Neonatal Lambs in the Alto Andino
- PMID: 35733986
- PMCID: PMC9207417
- DOI: 10.3389/fphys.2022.864010
Cinaciguat (BAY-582667) Modifies Cardiopulmonary and Systemic Circulation in Chronically Hypoxic and Pulmonary Hypertensive Neonatal Lambs in the Alto Andino
Abstract
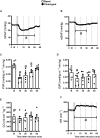
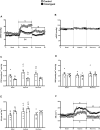
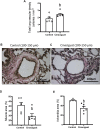
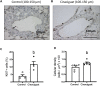
Neonatal pulmonary hypertension (NPHT) is produced by sustained pulmonary vasoconstriction and increased vascular remodeling. Soluble guanylyl cyclase (sGC) participates in signaling pathways that induce vascular vasodilation and reduce vascular remodeling. However, when sGC is oxidized and/or loses its heme group, it does not respond to nitric oxide (NO), losing its vasodilating effects. sGC protein expression and function is reduced in hypertensive neonatal lambs. Currently, NPHT is treated with NO inhalation therapy; however, new treatments are needed for improved outcomes. We used Cinaciguat (BAY-582667), which activates oxidized and/or without heme group sGC in pulmonary hypertensive lambs studied at 3,600 m. Our study included 6 Cinaciguat-treated (35 ug kg-1 day-1 x 7 days) and 6 Control neonates. We measured acute and chronic basal cardiovascular variables in pulmonary and systemic circulation, cardiovascular variables during a superimposed episode of acute hypoxia, remodeling of pulmonary arteries and changes in the right ventricle weight, vasoactive functions in small pulmonary arteries, and expression of NO-sGC-cGMP signaling pathway proteins involved in vasodilation. We observed a decrease in pulmonary arterial pressure and vascular resistance during the acute treatment. In contrast, the pulmonary pressure did not change in the chronic study due to increased cardiac output, resulting in lower pulmonary vascular resistance in the last 2 days of chronic study. The latter may have had a role in decreasing right ventricular hypertrophy, although the direct effect of Cinaciguat on the heart should also be considered. During acute hypoxia, the pulmonary vascular resistance remained low compared to the Control lambs. We observed a higher lung artery density, accompanied by reduced smooth muscle and adventitia layers in the pulmonary arteries. Additionally, vasodilator function was increased, and vasoconstrictor function was decreased, with modifications in the expression of proteins linked to pulmonary vasodilation, consistent with low pulmonary vascular resistance. In summary, Cinaciguat, an activator of sGC, induces cardiopulmonary modifications in chronically hypoxic and pulmonary hypertensive newborn lambs. Therefore, Cinaciguat is a potential therapeutic tool for reducing pulmonary vascular remodeling and/or right ventricular hypertrophy in pulmonary arterial hypertension syndrome.
Keywords: Cinaciguat; high altitude; hypoxia; newborn; pulmonary hypertension.
Copyright © 2022 Beñaldo, Araya-Quijada, Ebensperger, Herrera, Reyes, Moraga, Riquelme, Gónzalez-Candia, Castillo-Galán, Valenzuela, Serón-Ferré and Llanos.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Cinaciguat, a soluble guanylate cyclase activator, augments cGMP after oxidative stress and causes pulmonary vasodilation in neonatal pulmonary hypertension.Am J Physiol Lung Cell Mol Physiol. 2011 Nov;301(5):L755-64. doi: 10.1152/ajplung.00138.2010. Epub 2011 Aug 19. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21856817 Free PMC article.
-
Cinaciguat, a soluble guanylate cyclase activator, causes potent and sustained pulmonary vasodilation in the ovine fetus.Am J Physiol Lung Cell Mol Physiol. 2009 Aug;297(2):L318-25. doi: 10.1152/ajplung.00062.2009. Epub 2009 May 22. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19465519 Free PMC article.
-
Nitric Oxide-Independent Soluble Guanylate Cyclase Activation Improves Vascular Function and Cardiac Remodeling in Sickle Cell Disease.Am J Respir Cell Mol Biol. 2018 May;58(5):636-647. doi: 10.1165/rcmb.2017-0292OC. Am J Respir Cell Mol Biol. 2018. PMID: 29268036 Free PMC article.
-
Nitric oxide-independent stimulation of soluble guanylate cyclase with BAY 41-2272 in cardiovascular disease.Cardiovasc Drug Rev. 2007 Spring;25(1):30-45. doi: 10.1111/j.1527-3466.2007.00003.x. Cardiovasc Drug Rev. 2007. PMID: 17445086 Review.
-
Soluble guanylate cyclase stimulators in pulmonary hypertension.Handb Exp Pharmacol. 2013;218:279-313. doi: 10.1007/978-3-642-38664-0_12. Handb Exp Pharmacol. 2013. PMID: 24092345 Review.
Cited by
-
Methods for establishing animal models of persistent pulmonary hypertension of the newborn: A systematic literature analysis.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024 Sept 28;49(9):1477-1494. doi: 10.11817/j.issn.1672-7347.2024.230539. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 39931778 Free PMC article. Chinese, English.
-
Biomechanical effects of hemin and sildenafil treatments on the aortic wall of chronic-hypoxic lambs.Front Bioeng Biotechnol. 2024 Jul 3;12:1406214. doi: 10.3389/fbioe.2024.1406214. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39021365 Free PMC article.
-
Biomechanical and histomorphometric characterization of the melatonin treatment effect in the carotid artery subjected to hypobaric hypoxia.Front Bioeng Biotechnol. 2025 Apr 16;13:1554004. doi: 10.3389/fbioe.2025.1554004. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40309506 Free PMC article.
-
Decoding signaling mechanisms: unraveling the targets of guanylate cyclase agonists in cardiovascular and digestive diseases.Front Pharmacol. 2023 Dec 20;14:1272073. doi: 10.3389/fphar.2023.1272073. eCollection 2023. Front Pharmacol. 2023. PMID: 38186653 Free PMC article. Review.
References
-
- Acker S. N., Seedorf G. J., Abman S. H., Nozik-Grayck E., Partrick D. A., Gien J. (2013). Pulmonary Artery Endothelial Cell Dysfunction and Decreased Populations of Highly Proliferative Endothelial Cells in Experimental Congenital Diaphragmatic Hernia. Am. J. Physiology-Lung Cell. Mol. Physiology 305 (12), L943–L952. 10.1152/ajplung.00226.2013 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

