Severe Acute Respiratory Syndrome Coronavirus 2 Antibody Response After Heterologous Immunizations With ChAdOx1/BNT162b2 in End-Stage Renal Disease Patients on Hemodialysis
- PMID: 35734170
- PMCID: PMC9207316
- DOI: 10.3389/fimmu.2022.894700
Severe Acute Respiratory Syndrome Coronavirus 2 Antibody Response After Heterologous Immunizations With ChAdOx1/BNT162b2 in End-Stage Renal Disease Patients on Hemodialysis
Abstract
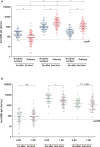
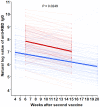
The Korean government decided to schedule heterologous vaccinations on dialysis patients for early achievement of immunization against Coronavirus disease 2019(COVID-19). However, the effects of heterologous immunizations in hemodialysis (HD) patients are unclear. One hundred (HD) patients from Gangdong Kyung Hee University Hospital and Kyung Hee Medical Center and 100 hospital workers from Gangdong Kyung Hee University Hospital were enrolled in this study. The HD patients received the mixing schedule of ChAdOx1/BNT162b2 vaccinations at 10-week intervals, while hospital workers received two doses of ChAdOx1 vaccines at 12-week intervals. Serum IgG to a receptor-binding domain (RBD) of the S1 subunit of the spike protein of SARS-CoV-2 was measured 1 month after the first dose, 2 months and 4 months after the second dose. The median [interquartile range] anti-RBD IgG was 82.1[34.5, 176.6] AU/ml in HD patients and 197.1[124.0, 346.0] AU/ml in hospital workers (P < 0.001) after the first dose. The percentage of positive responses (IgG > 50 AU/ml) was 65.0% and 96.0% among the both group, respectively (P < 0.001). The anti-RBD IgG levels increased significantly by 2528.8 [1327.6, 5795.1] AU/ml with a 100.0% positive response rate in HD patients 2 months after the second dose, which was higher than those in hospital workers 981.4[581.5, 1891.4] AU/ml (P < 0.001). Moreover, anti-RBD IgG remains constantly high, and positive response remains 100% in HD patients 4 months after the second dose. This study suggests that heterologous vaccinations with ChAdOx1/BNT162b2 can be an alternative solution on HD patients for early and strong induction of humoral response.
Keywords: BNT162b2; COVID-19; ChAdOx1; anti-RBD IgG; hemodialysis; heterologous vaccination.
Copyright © 2022 Kim, Jung, Moon, Jeong, Hwang, Kim, Lee, Kang and Kim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- English E, Cook LE, Piec I, Dervisevic S, Fraser WD, John WG. Performance of the Abbott Sars-Cov-2 Igg Ii Quantitative Antibody Assay Including the New Variants of Concern, Voc 202012/V1 (United Kingdom) and Voc 202012/V2 (South Africa), and First Steps Towards Global Harmonization of Covid-19 Antibody Methods. J Clin Microbiol (2021) 59(9):e0028821. doi: 10.1128/JCM.00288-21 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

