Current research in pathophysiology of opioid-induced respiratory depression, neonatal opioid withdrawal syndrome, and neonatal antidepressant exposure syndrome
- PMID: 35734228
- PMCID: PMC9207297
- DOI: 10.1016/j.crtox.2022.100078
Current research in pathophysiology of opioid-induced respiratory depression, neonatal opioid withdrawal syndrome, and neonatal antidepressant exposure syndrome
Abstract
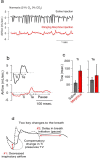
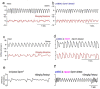
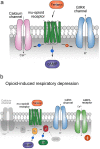
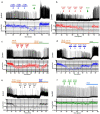
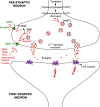
Respiratory depression (RD) is the primary cause of death due to opioids. Opioids bind to mu (µ)-opioid receptors (MORs) encoded by the MOR gene Oprm1, widely expressed in the central and peripheral nervous systems including centers that modulate breathing. Respiratory centers are located throughout the brainstem. Experiments with Oprm1-deleted knockout (KO) mice undertaken to determine which sites are necessary for the induction of opioid-induced respiratory depression (OIRD) showed that the pre-Bötzinger complex (preBötC) and the pontine Kölliker-Fuse nucleus (KF) contribute equally to OIRD but RD was not totally eliminated. Morphine showed a differential influence on preBötC and KF neurons - low doses attenuated RD following deletion of MORs from preBötC neurons and an increase in apneas after high doses whereas deletion of MORs from KF neurons but not the preBötC attenuated RD at both high and low doses. In other KO mice studies, morphine administration after deletion of Oprm1 from both the preBötC and the KF/PBN neurons, led to the conclusion that both respiratory centres contribute to OIRD but the preBötC predominates. MOR-mediated post-synaptic activation of GIRK potassium channels has been implicated as a cause of OIRD. A complementary mechanism in the preBötC involving KCNQ potassium channels independent of MOR signaling has been described. Recent experiments in rats showing that morphine depresses normal, but not gasping breathing, cast doubt on the belief that eupnea, sighs, and gasps, are under the control of preBötC neurons. Methadone, administered to alleviate symptoms of neonatal opioid withdrawal syndrome (NOWES), desensitized rats to OIRD. Protection lost between postnatal days 1 and 2 coincides with the preBötC becoming the dominant generator of respiratory rhythm. Neonatal antidepressant exposure syndrome (NADES) and serotonin toxicity (ST) show similarities including RD. Enzyme CYP2D6 involved in opioid detoxification is polymorphic. Individuals of different CYP2D6 genotype may show increased, decreased, or no enzyme activity, contributing to the variability of patient responses to different opioids and OIRD.
Keywords: AAV, adeno-associated virus; CDC, Centers for Disease control and prevention; CTAP, MOR agonist (D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2); DAMGO, synthetic specific MOR agonist [D-Ala2, N-MePhe4, Gly-ol]-enkephalin; DRG, dorsal respiratory group; FDA, Food and Drug Administration; GIRK, G protein-gated inwardly-rectifying potassium (K+); GPCR, G protein-coupled receptor; KCNQ, voltage-gated potassium (Kv) channels in the KCNQ (Kv7) family; KF, Kölliker-Fuse nucleus; Kölliker-Fuse nucleus and opioid-induced respiratory depression; MOR, mu opioid receptor; NADES, neonatal antidepressant exposure syndrome; NAS, neonatal abstinence syndrome; NIH, National Institutes of Health; NK-1R, neurokinin-1 receptor; NOWES, neonatal opioid withdrawal syndrome; Neonatal opioid withdrawal syndrome; Neural mediation of opioid-induced respiratory depression; OAD, opioid analgesic drug; OIRD, opioid-induced respiratory depression; PBL, lateral parabrachial; PBN, parabrachial nucleus; PRG, pontine respiratory group; Pathophysiology of opioid-induced respiratory depression; Pre-Bötzinger complex and opioid-induced respiratory depression; RD, respiratory depression; TACR1, tachykinin receptor 1; VRG, ventral respiratory group; preBötC, pre-Bötzinger complex.
© 2022 Published by Elsevier B.V.
Conflict of interest statement
Brian A. Baldo has no support to declare and there are no other relationships or activities of any kind to declare.
Figures



References
-
- Algera M.H., Kamp J., van der Schrier R., van Velzen M., Niesters M., Aarts L., Dahan A., Olofsen E. Opioid-induced respiratory depression in humans: a review of pharmacokinetic-pharmacodynamic modelling of reversal. Br. J. Anaesth. 2019;122(6):e168–e179. doi: 10.1016/j.bja.2018.12.023. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

