Synthesis and Characterization of Novel [2 + 1] Tricarbonyl Rhenium Complexes with the Hydrophilic Phosphine Ligands PTA and CAP
- PMID: 35734344
- PMCID: PMC9208990
- DOI: 10.1155/2022/3117661
Synthesis and Characterization of Novel [2 + 1] Tricarbonyl Rhenium Complexes with the Hydrophilic Phosphine Ligands PTA and CAP
Abstract
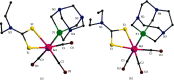
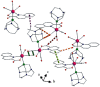
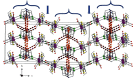
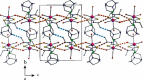
In the pursuit of hydrophilic model fac-[Re(CO)3]+ complexes for (radio) pharmaceutical applications, six novel [2 + 1] mixed-ligand complexes of the general type fac-[Re(CO)3(bid)P] were synthesized and characterized, where bid is a bidentate ligand bearing either (N, O) or (S, S') donor atom sets and P is the hydrophilic phosphine 1,3,5-triaza-7-phosphoadamantane (PTA) or its macrocyclic homologue 1,4,7-triaza-9-phosphatricyclo[5.3.2.1]tridecane (CAP). The (N, O) ligands used in this study were picolinic and quinaldic acid, while the (S, S') ligand was diethyldithiocarbamate. The complexes were synthesized in generally high yields and purity and the characterization was performed by spectroscopic methods, IR, NMR, and elemental analysis. Detailed X-ray crystallographic study of molecular packing by using Hirshfeld analysis tools revealed a plethora of intermolecular interactions such as hydrogen bond, π⋯π, C-H⋯π, and carbonyl-carbonyl interactions. To our knowledge, the CAP complexes reported herein are the first example of [2 + 1] mixed-ligand fac-[Re(CO)3]+ complexes with CAP. The new complexes might have the potential to serve as platforms for the design of target-specific complexes with favorable pharmacokinetics.
Copyright © 2022 Ioanna Roupa et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures







Similar articles
-
Synthesis, characterization, kinetic investigation and biological evaluation of Re(i) di- and tricarbonyl complexes with tertiary phosphine ligands.Dalton Trans. 2020 Jan 7;49(1):35-46. doi: 10.1039/c9dt04025k. Epub 2019 Nov 19. Dalton Trans. 2020. PMID: 31740907
-
Tuning the reactivity in classic low-spin d6 rhenium(I) tricarbonyl radiopharmaceutical synthon by selective bidentate ligand variation (L,L'-Bid; L,L'= N,N', N,O, and O,O' donor atom sets) in fac-[Re(CO)3(L,L'-Bid)(MeOH)]n complexes.Inorg Chem. 2011 Dec 19;50(24):12486-98. doi: 10.1021/ic2013792. Epub 2011 Nov 23. Inorg Chem. 2011. PMID: 22111710
-
Synthesis and evaluation of new mixed "2 + 1" Re, 99mTc and 186Re tricarbonyl dithiocarbamate complexes with different monodentate ligands.Bioorg Med Chem. 2021 Oct 1;47:116373. doi: 10.1016/j.bmc.2021.116373. Epub 2021 Aug 19. Bioorg Med Chem. 2021. PMID: 34467870
-
Synthesis and characterization of fac-[M(CO)3(P)(OO)] and cis-trans-[M(CO)2(P)2(OO)] complexes (M = Re, (99m)Tc) with acetylacetone and curcumin as OO donor bidentate ligands.Inorg Chem. 2013 Nov 18;52(22):12995-3003. doi: 10.1021/ic401503b. Epub 2013 Nov 7. Inorg Chem. 2013. PMID: 24199833
-
Rhenium-Pyrazolyl-Based Figure-Eight- and Z-Shaped Metallocycles: Self-Assembly, Solid-State Structures, Dynamic Properties in Solution, and Competitive Ligand-Induced Supramolecular Transformations into Rhenium-Pyridyl/-Benzimidazolyl/-Phosphine-Based Metallocycles/Acyclic Complexes.ACS Omega. 2023 Oct 30;8(44):41773-41784. doi: 10.1021/acsomega.3c06371. eCollection 2023 Nov 7. ACS Omega. 2023. PMID: 37969972 Free PMC article.
Cited by
-
Synthesis and Evaluation of 99mTc(CO)3 Complexes with Ciprofloxacin Dithiocarbamate for Infection Imaging.Pharmaceutics. 2024 Sep 14;16(9):1210. doi: 10.3390/pharmaceutics16091210. Pharmaceutics. 2024. PMID: 39339246 Free PMC article.
References
-
- Abram U., Alberto R. Technetium and rhenium: coordination chemistry and nuclear medical applications. Journal of the Brazilian Chemical Society . 2006;17(8):1486–1500. doi: 10.1590/s0103-50532006000800004. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

