Potency and Therapeutic THC and CBD Ratios: U.S. Cannabis Markets Overshoot
- PMID: 35734402
- PMCID: PMC9207456
- DOI: 10.3389/fphar.2022.921493
Potency and Therapeutic THC and CBD Ratios: U.S. Cannabis Markets Overshoot
Abstract
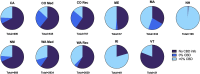
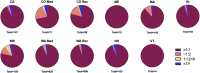
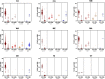
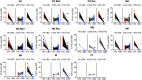
Background and aims: The effects exuded by cannabis are a result of the cannabinoids trans-Δ⁹-tetrahydrocannabinol (THC) and cannabidiol (CBD), and is dependent upon their pharmacological interaction and linked to the two cannabinoids' concentrations and ratios. Based on current literature and trends of increasing cannabis potency, we postulate that most medical cannabis products with THC and CBD have ratios capable of producing significant acute intoxication and are similar to recreational products. We will test this by organizing products into clinically distinct categories according to TCH:CBD ratios, evaluating the data in terms of therapeutic potential, and comparing the data obtained from medical and recreational programs and from states with differing market policies. Methods: We utilized data encompassing online herbal dispensary product offerings from nine U.S. states. The products were analyzed after being divided into four clinically significant THC:CBD ratio categories identified based on the literature: CBD can enhance THC effects (THC:CBD ratios ≥1:1), CBD has no significant effect on THC effects (ratios ∼ 1:2), CBD can either have no effect or can mitigate THC effects (ratios 1:>2 < 6), or CBD is protective against THC effects (ratios ≤1:6). Results: A significant number of products (58.5%) did not contain any information on CBD content. Across all states sampled, the majority (72-100%) of both medical and recreational products with CBD (>0%) fall into the most intoxicating ratio category (≥1:1 THC:CBD), with CBD likely enhancing THC's acute effects. The least intoxicating categories (1:>2 < 6 and ≤1:6 THC:CBD) provided the smallest number of products. Similarly, the majority of products without CBD (0%) contained highly potent amounts of THC (>15%). These results were consistent, regardless of differing market policies in place. Conclusions: Despite the distinct goals of medical and recreational cannabis users, medical and recreational program product offerings are nearly identical. Patients seeking therapeutic benefits from herbal cannabis products are therefore at a substantial risk of unwanted side effects, regardless of whether they obtain products from medical or recreational programs. Efforts are needed to better inform patients of the risks associated with high potency cannabis and the interaction between THC and CBD, and to help shape policies that promote more therapeutic options.
Keywords: cannabidiol; cannabis market; herbal cannabis; intoxication; marijuana; medical marijuana; potency; tetrahydrocannabinol.
Copyright © 2022 Pennypacker, Cunnane, Cash and Romero-Sandoval.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Arkell T. R., Lintzeris N., Kevin R. C., Ramaekers J. G., Vandrey R., Irwin C., et al. (2019). Cannabidiol (CBD) Content in Vaporized Cannabis Does Not Prevent Tetrahydrocannabinol (THC)-induced Impairment of Driving and Cognition. Psychopharmacol. Berl. 236 (9), 2713–2724. 10.1007/s00213-019-05246-8 - DOI - PMC - PubMed
-
- Bergamaschi M. M., Queiroz R. H., Chagas M. H., de Oliveira D. C., De Martinis B. S., Kapczinski F., et al. (2011a). Cannabidiol Reduces the Anxiety Induced by Simulated Public Speaking in Treatment-Naïve Social Phobia Patients. Neuropsychopharmacology 36 (6), 1219–1226. 10.1038/npp.2011.6 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

