Polyclonal antibody preparations from avian origin as a feed additive to beef cattle: ruminal fermentation during the step-up transition diets
- PMID: 35734554
- PMCID: PMC9206718
- DOI: 10.1093/tas/txac070
Polyclonal antibody preparations from avian origin as a feed additive to beef cattle: ruminal fermentation during the step-up transition diets
Abstract
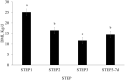
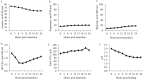
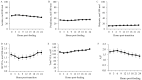
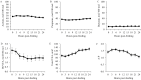
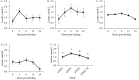
This study investigated the effects of feeding an avian-derived polyclonal antibody preparation (PAP; CAMAS, Inc.) against Streptococcus bovis, Fusobacterium necrophorum, and lipopolysaccharides (40%, 35%, and 25% of the preparation, respectively) on ruminal fermentation [pH, ammonia-N (NH3-N), lactate, and volatile fatty acids (VFA)] of beef steers during a 21-d step-up diet adaptation. Eight ruminally cannulated Angus crossbred beef steers (658 ± 79 kg of body weight) were assigned in a crossover design to be transitioned from a diet containing ad libitum bermudagrass hay [Cynodon dactylon (L.) Pers.] plus 0.45 kg/d (as fed) of molasses with 0 (CON) or 3 g of PAP (PAP) to a high-grain diet. Transition consisted of three 7-d steps of increased inclusion of cracked corn (35%, 60%, and 82% of the diet DM for STEP1, STEP2, and STEP3, respectively). On each transition day and 7 d after STEP3 (STEP3-7d), ruminal fluid samples were obtained every 3 h for 24 h. Feeding 3 g of PAP daily increased (P < 0.01) average ruminal pH during STEP3 compared with CON steers (5.6 vs. 5.4 ± 0.05, respectively). During STEP1, NH3-N concentration was greater (P < 0.01; 9.4 vs. 6.8 ± 0.74 mM, respectively), and time (min/d) and area (time × pH) of ruminal pH below or equal to 5.2 was lesser (P ≤ 0.03) for steers consuming PAP compared with steers assigned to CON treatment (33.4 vs. 73.3 ± 21.7 min/d and 187.4 vs. 406.3 ± 119.7 min × pH/d, respectively). Steers consuming PAP had greater acetate:propionate ratio at 0, 3, and 6 h relative to diet change compared with CON (2.42, 2.35, 2.29 vs. 1.66, 1.79, and 1.72 ± 0.17, respectively), whereas butyrate molar proportions increased (P = 0.02; 17.1 vs. 11 ± 1.58 mol/100 mol for CON and PAP, respectively) when PAP was not fed at STEP2. Total ruminal lactate concentrations were not affected by PAP feeding (P > 0.11). In conclusion, feeding 3 g/d of polyclonal antibody preparation against S. bovis, F. necrophorum, and lipopolysaccharides was effective in increasing ruminal pH, A:P ratio, and NH3-N concentrations, possibly attenuating the risks of ruminal acidosis in steers during the step-up transition from forage to high-grain diets.
Keywords: Fusobacterium necrophorum; Streptococcus bovis; lipopolysaccharides; step-up process.
© The Author(s) 2022. Published by Oxford University Press on behalf of the American Society of Animal Science.
Figures



References
-
- Asanuma, N., and Hino T.. . 2005. Ability to utilize lactate and related enzymes of a ruminal bacterium, Selenomonas ruminantium. Anim. Sci. J. 76:345–352. doi: 10.1111/j.1740-0929.2005.00274.x - DOI
-
- Barros, T. A., Cassiano E. C. O., Marino C. T., Pacheco R. D. L., Ferreira F. A., Junior F. P., Martins M. F., Meyer P. M., Millen D. D., Arrigoni M. D. B., . et al. 2019. Polyclonal antibodies as a feed additive for cattle adapted or not adapted to highly fermentable carbohydrate diets. J. Appl. Anim. Res. 47:565–572. doi: 10.1080/09712119.2019.1691561 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
