Host SUMOylation Pathway Negatively Regulates Protective Immune Responses and Promotes Leishmania donovani Survival
- PMID: 35734580
- PMCID: PMC9207379
- DOI: 10.3389/fcimb.2022.878136
Host SUMOylation Pathway Negatively Regulates Protective Immune Responses and Promotes Leishmania donovani Survival
Abstract
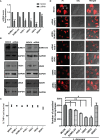
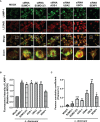
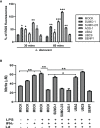
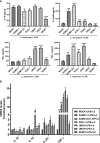
SUMOylation is one of the post-translational modifications that have recently been described as a key regulator of various cellular, nuclear, metabolic, and immunological processes. The process of SUMOylation involves the modification of one or more lysine residues of target proteins by conjugation of a ubiquitin-like, small polypeptide known as SUMO for their degradation, stability, transcriptional regulation, cellular localization, and transport. Herein, for the first time, we report the involvement of the host SUMOylation pathway in the process of infection of Leishmania donovani, a causative agent of visceral leishmaniasis. Our data revealed that infection of L. donovani to the host macrophages leads to upregulation of SUMOylation pathway genes and downregulation of a deSUMOylating gene, SENP1. Further, to confirm the effect of the host SUMOylation on the growth of Leishmania, the genes associated with the SUMOylation pathway were silenced and parasite load was analyzed. The knockdown of the SUMOylation pathway led to a reduction in parasitic load, suggesting the role of the host SUMOylation pathway in the disease progression and parasite survival. Owing to the effect of the SUMOylation pathway in autophagy, we further investigated the status of host autophagy to gain mechanistic insights into how SUMOylation mediates the regulation of growth of L. donovani. Knockdown of genes of host SUMOylation pathway led to the reduction of the expression levels of host autophagy markers while promoting autophagosome-lysosome fusion, suggesting SUMOylation-mediated autophagy in terms of autophagy initiation and autophagy maturation during parasite survival. The levels of reactive oxygen species (ROS) generation, nitric oxide (NO) production, and pro-inflammatory cytokines were also elevated upon the knockdown of genes of the host SUMOylation pathway during L. donovani infection. This indicates the involvement of the SUMOylation pathway in the modulation of protective immune responses and thus favoring parasite survival. Taken together, the results of this study indicate the hijacking of the host SUMOylation pathway by L. donovani toward the suppression of host immune responses and facilitation of host autophagy to potentially facilitate its survival. Targeting of SUMOylation pathway can provide a starting point for the design and development of novel therapeutic interventions to combat leishmaniasis.
Keywords: Leishmania donavani; SUMOylation; SUMOylation mediated immune responses; autophagy; autophagy maturation; host–pathogen interaction.
Copyright © 2022 Singhal, Madan, Chaurasiya, Srivastava, Singh, Kaushik, Kahlon, Maurya, Marothia, Joshi, Ranganathan and Singh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

