Effectiveness and Safety of Therapeutic Vaccines for Precancerous Cervical Lesions: A Systematic Review and Meta-Analysis
- PMID: 35734598
- PMCID: PMC9207463
- DOI: 10.3389/fonc.2022.918331
Effectiveness and Safety of Therapeutic Vaccines for Precancerous Cervical Lesions: A Systematic Review and Meta-Analysis
Abstract
Objective: This study systematically evaluated the effectiveness and safety of therapeutic vaccines for precancerous cervical lesions, providing evidence for future research.
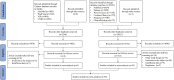
Methods: We systematically searched the literature in 10 databases from inception to February 18, 2021. Studies on the effectiveness and safety of therapeutic vaccines for precancerous cervical lesions were included. Then, we calculated the overall incidence rates of four outcomes, for which we used the risk ratio (RR) and 95% confidence interval (95% CI) to describe the effects of high-grade squamous intraepithelial lesions (HSILs) on recurrence.
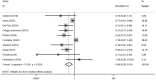
Results: A total of 39 studies were included, all reported in English, published from 1989 to 2021 in 16 countries. The studies covered 22,865 women aged 15-65 years, with a total of 5,794 vaccinated, and 21 vaccines were divided into six types. Meta-analysis showed that the overall incidence rate of HSIL regression in vaccine therapies was 62.48% [95% CI (42.80, 80.41)], with the highest rate being 72.32% for viral vector vaccines [95% CI (29.33, 99.51)]. Similarly, the overall incidence rates of HPV and HPV16/18 clearance by vaccines were 48.59% [95% CI (32.68, 64.64)] and 47.37% [95% CI (38.00, 56.81)], respectively, with the highest rates being 68.18% [95% CI (45.13, 86.14)] for bacterial vector vaccines and 55.14% [95% CI (42.31, 67.66)] for DNA-based vaccines. In addition, a comprehensive analysis indicated that virus-like particle vaccines after conization reduced the risk of HSIL recurrence with statistical significance compared to conization alone [RR = 0.46; 95% CI (0.29, 0.74)]. Regarding safety, only four studies reported a few severe adverse events, indicating that vaccines for precancerous cervical lesions are generally safe.
Conclusion: Virus-like particle vaccines as an adjuvant immunotherapy for conization can significantly reduce the risk of HSIL recurrence. Most therapeutic vaccines have direct therapeutic effects on precancerous lesions, and the effectiveness in HSIL regression, clearance of HPV, and clearance of HPV16/18 is great with good safety. That is, therapeutic vaccines have good development potential and are worthy of further research.
Systematic review registration: PROSPERO https://www.crd.york.ac.uk/PROSPERO/, CRD42021275452.
Keywords: effectiveness; human papillomavirus; precancerous cervical lesions; safety; therapeutic vaccines.
Copyright © 2022 Cai, Tan, Miao, Li, Cheng, Li, Zeng and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- World Health Organization . Estimated Number of Incident Cases and Deaths Worldwide, Females, All Ages . Available at: https://gco.iarc.fr (Accessed 29 January 2022).
-
- World Health Organization . Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem . Available at: https://www.who.int/publications/i/item/9789240014107 (Accessed 15 February 2022).
-
- World Health Organization . Comprehensive Cervical Cancer Control: A Guide to Essential Practice - Second Edition . Available at: https://www.who.int/reproductivehealth/publications/cancers/cervical-can... (Accessed 15 February 2022). - PubMed
-
- World Health Organization . WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention, Second Edition: Use of mRNA Tests for Human Papillomavirus (HPV) . Available at: https://www.who.int/publications/i/item/9789240040434 (Accessed 15 February 2022). - PubMed
-
- Li T, Wang C. High Risk HPV Test Combined With TCT in the Screening of Cervical Intraepithelial Neoplasia. Chin Gen Pract (2021) 24(09):1106–10. doi: 10.12114/j.issn.1007-9572.2021.00.443 - DOI
Publication types
LinkOut - more resources
Full Text Sources