In vitro dynamic perfusion of prevascularized OECs-DBMs (outgrowth endothelial progenitor cell - demineralized bone matrix) complex fused to recipient vessels in an internal inosculation manner
- PMID: 35734812
- PMCID: PMC9342144
- DOI: 10.1080/21655979.2022.2085560
In vitro dynamic perfusion of prevascularized OECs-DBMs (outgrowth endothelial progenitor cell - demineralized bone matrix) complex fused to recipient vessels in an internal inosculation manner
Abstract
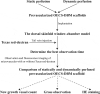
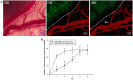
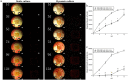
The current research on seed cells and scaffold materials of bone tissue engineering has achieved milestones. Nevertheless, necrosis of seed cells in center of bone scaffold is a bottleneck in tissue engineering. Therefore, this study aimed to investigate the in vivo inosculation mechanism of recipient microvasculature and prevascularized outgrowth endothelial progenitor cells (OECs)-demineralized bone matrix (DBM) complex. A dorsal skinfold window-chamber model with tail vein injection of Texas red-dextran was established to confirm the optimal observation time of microvessels. OECs-DBM complex under static and dynamic perfusion culture was implanted into the model to analyze vascularization. OECs-DBM complex was harvested on 12th day for HE staining and fluorescent imaging. The model was successfully constructed, and the most appropriate time to observe microvessels was 15 min after injection. The ingrowth of recipient microvessels arcoss the border of OECs-DBM complex increased with time in both groups, and more microvessels across the border were observed in dynamic perfusion group on 3rd, 5th, 7th day. Fluorescent integrated density of border in dynamic perfusion group was higher at all-time points, and the difference was more significant in central area. Fluorescent imaging of OECs-DBM complex exhibited that no enhanced green fluorescent protein-positive cells were found beyond the verge of DBM scaffold in both groups. In vitro prevascularization by dynamic perfusion culture can increase and accelerate the blood perfusion of OECs-DBM complex obtained from recipient microvasculature by internal inosculation. Accordingly, this approach may markedly contribute to the future success of tissue engineering applications in clinical practice.
Keywords: Dynamic perfusion culture; decalcified bone matrix; internal inosculation; outgrowth endothelial progenitor cells; vascularization.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures






Similar articles
-
Dynamic Perfusion Culture of Human Outgrowth Endothelial Progenitor Cells on Demineralized Bone Matrix In Vitro.Med Sci Monit. 2016 Oct 28;22:4037-4045. doi: 10.12659/msm.897884. Med Sci Monit. 2016. PMID: 27789903 Free PMC article.
-
Short-term cultivation of in situ prevascularized tissue constructs accelerates inosculation of their preformed microvascular networks after implantation into the host tissue.Tissue Eng Part A. 2011 Mar;17(5-6):841-53. doi: 10.1089/ten.TEA.2010.0329. Epub 2010 Dec 7. Tissue Eng Part A. 2011. PMID: 20973748
-
Dynamic compression promotes proliferation and neovascular networks of endothelial progenitor cells in demineralized bone matrix scaffold seed.J Appl Physiol (1985). 2012 Aug 15;113(4):619-26. doi: 10.1152/japplphysiol.00378.2011. Epub 2012 Jun 21. J Appl Physiol (1985). 2012. PMID: 22723630
-
Endothelial Progenitor Cells for the Vascularization of Engineered Tissues.Tissue Eng Part B Rev. 2018 Feb;24(1):1-24. doi: 10.1089/ten.TEB.2017.0127. Epub 2017 Jul 3. Tissue Eng Part B Rev. 2018. PMID: 28548628 Free PMC article. Review.
-
Natural Sources and Applications of Demineralized Bone Matrix in the Field of Bone and Cartilage Tissue Engineering.Adv Exp Med Biol. 2020;1249:3-14. doi: 10.1007/978-981-15-3258-0_1. Adv Exp Med Biol. 2020. PMID: 32602087 Review.
Cited by
-
Research Progress of Bone Grafting: A Comprehensive Review.Int J Nanomedicine. 2025 Apr 15;20:4729-4757. doi: 10.2147/IJN.S510524. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40255675 Free PMC article. Review.
References
-
- Khojasteh A, Fahimipour F, Eslaminejad MB, et al. Development of PLGA-coated β-TCP scaffolds containing VEGF for bone tissue engineering. Mater Sci Eng C Mater Bio Appl. 2016;69:780–788. - PubMed
-
- Dou DD, Zhou G, Liu HW, et al. Sequential releasing of VEGF and BMP-2 in hydroxyapatite collagen scaffolds for bone tissue engineering: design and characterization. Int J Biol Macromol. 2019;123:622–628. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
