Clearance of atypical cutaneous manifestations of hyper-IgE syndrome with dupilumab
- PMID: 35734823
- PMCID: PMC10084161
- DOI: 10.1111/pde.15072
Clearance of atypical cutaneous manifestations of hyper-IgE syndrome with dupilumab
Abstract
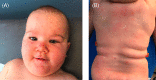
Hyper-IgE syndromes (HIES) are a heterogeneous group of rare primary immunodeficiency diseases classically characterized by the triad of atopic dermatitis, and recurrent cutaneous and pulmonary infections. Autosomal dominant, loss-of-function STAT3 pathogenic variants are the most common genetic cause, which lead to deficiency of Th17 lymphocytes, impaired interferon gamma production, and IL-10 signal transduction, and an unbalanced IL-4 state. Dupilumab, a monoclonal antibody to the IL-4a receptor, inhibits both IL-4 and IL-13, and has been shown to improve atopic dermatitis and other manifestations of HIES including asthma and allergic bronchopulmonary aspergillosis. We present a pediatric patient with HIES who presented predominantly with eosinophilic folliculitis, recurrent cutaneous infections, and other non-eczematous findings and achieved sustained clearance with dupilumab.
Keywords: atopic dermatitis; dupilumab; eczema; genetic syndromes; hyper-IgE syndrome.
© 2022 The Authors. Pediatric Dermatology published by Wiley Periodicals LLC.
Conflict of interest statement
Kristen E. Holland served as an investigator for Pfizer, Amgen, Incyte, Abbvie, and Sanofi. Abbvie also provides spouse salary. Anne Marie Singh has consulted for Abbvie and currently serves on the data monitoring board for Siolta Therapeutics. She receives research funding from the NIH. Lisa M. Arkin has consulted for Abbvie and Regeneron. The remaining authors declare no conflicts of interest.
Figures



References
-
- Hafsi W, Yarrarapu SNS. Job Syndrome. StatPearls Publishing; 2021. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

