C3 glomerulopathy: Understanding an ultra-rare complement-mediated renal disease
- PMID: 35734939
- PMCID: PMC9613507
- DOI: 10.1002/ajmg.c.31986
C3 glomerulopathy: Understanding an ultra-rare complement-mediated renal disease
Abstract
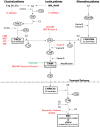
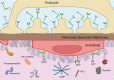
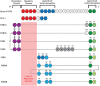
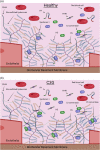
C3 glomerulopathy (C3G) describes a pathologic pattern of injury diagnosed by renal biopsy. It is characterized by the dominant deposition of the third component of complement (C3) in the renal glomerulus as resolved by immunofluorescence microscopy. The underlying pathophysiology is driven by dysregulation of the alternative pathway of complement in the fluid-phase and in the glomerular microenvironment. Characterization of clinical features and a targeted evaluation for indices and drivers of complement dysregulation are necessary for optimal patient care. Autoantibodies to the C3 and C5 convertases of complement are the most commonly detected drivers of complement dysregulation, although genetic mutations in complement genes can also be found. Approximately half of patients progress to end-stage renal disease within 10 years of diagnosis, and, while transplantation is a viable option, there is high risk for disease recurrence and allograft failure. This poor outcome reflects the lack of disease-specific therapy for C3G, relegating patients to symptomatic treatment to minimize proteinuria and suppress renal inflammation. Fortunately, the future is bright as several anti-complement drugs are currently in clinical trials.
Keywords: C3 glomerulonephritis; C3 glomerulopathy; dense deposit disease.
© 2022 The Authors. American Journal of Medical Genetics Part C: Seminars in Medical Genetics published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures


References
-
- Avasare, R. S. , Canetta, P. A. , Bomback, A. S. , Marasa, M. , Caliskan, Y. , Ozluk, Y. , … Appel, G. B. (2018). Mycophenolate mofetil in combination with steroids for treatment of C3 glomerulopathy: A case series. Clinical Journal of the American Society of Nephrology, 13(3), 406–413. 10.2215/CJN.09080817 - DOI - PMC - PubMed
-
- Blackmore, T. K. , Hellwage, J. , Sadlon, T. A. , Higgs, N. , Zipfel, P. F. , Ward, H. M. , & Gordon, D. L. (1998). Identification of the second heparin‐binding domain in human complement factor H. Journal of Immunology, 160(7), 3342–3348. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

