Abnormal white-matter rich-club organization in obsessive-compulsive disorder
- PMID: 35735129
- PMCID: PMC9491289
- DOI: 10.1002/hbm.25984
Abnormal white-matter rich-club organization in obsessive-compulsive disorder
Abstract
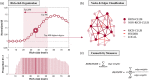
Rich-club organization is key to efficient global neuronal signaling and integration of information. Alterations interfere with higher-order cognitive processes, and are common to several psychiatric and neurological conditions. A few studies examining the structural connectome in obsessive-compulsive disorder (OCD) suggest lower efficiency of information transfer across the brain. However, it remains unclear whether this is due to alterations in rich-club organization. In the current study, the structural connectome of 28 unmedicated OCD patients, 8 of their unaffected siblings and 28 healthy controls was reconstructed by means of diffusion-weighted imaging and probabilistic tractography. Topological and weighted measures of rich-club organization and connectivity were computed, alongside global and nodal measures of network integration and segregation. The relationship between clinical scores and network properties was explored. Compared to healthy controls, OCD patients displayed significantly lower topological and weighted rich-club organization, allocating a smaller fraction of all connection weights to the rich-club core. Global clustering coefficient, local efficiency, and clustering of nonrich club nodes were significantly higher in OCD patients. Significant three-group differences emerged, with siblings displaying highest and lowest values in different measures. No significant correlation with any clinical score was found. Our results suggest weaker structural connectivity between rich-club nodes in OCD patients, possibly resulting in lower network integration in favor of higher network segregation. We highlight the need of looking at network-based alterations in brain organization and function when investigating the neurobiological basis of this disorder, and stimulate further research into potential familial protective factors against the development of OCD.
Keywords: connectivity; diffusion-weighted imaging; obsessive-compulsive disorder; probabilistic tractography; rich-club organization; structural networks.
© 2022 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
Odile A. van den Heuvel received a one‐time consultation fee (2021) from Lundbeck. The other authors declare that they have no conflict of interest.
Figures

References
-
- Association, A. P. , & Association, A. P. (2013). Diagnostic and statistical manual of mental disorders: DSM‐5. Arlington.
-
- Baggio, H. C. , Segura, B. , Junque, C. , de Reus, M. A. , Sala‐Llonch, R. , & van den Heuvel, M. P. (2015). Rich club organization and cognitive performance in healthy older participants. Journal of Cognitive Neuroscience, 27(9), 1801–1810. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

