Overdetection of Breast Cancer
- PMID: 35735420
- PMCID: PMC9222123
- DOI: 10.3390/curroncol29060311
Overdetection of Breast Cancer
Abstract
Overdetection (often referred to as overdiagnosis) of cancer is the detection of disease, such as through a screening program, that would otherwise remain occult through an individual's life. In the context of screening, this could occur for cancers that were slow growing or indolent, or simply because an unscreened individual would have died from some other cause before the cancer had surfaced clinically. The main harm associated with overdetection is the subsequent overdiagnosis and overtreatment of disease. In this article, the phenomenon is reviewed, the methods of estimation of overdetection are discussed and reasons for variability in such estimates are given, with emphasis on an analysis using Canadian data. Microsimulation modeling is used to illustrate the expected time course of cancer detection that gives rise to overdetection. While overdetection exists, the actual amount is likely to be much lower than the estimate used by the Canadian Task Force on Preventive Health Care. Furthermore, the issue is of greater significance in older rather than younger women due to competing causes of death. The particular challenge associated with in situ breast cancer is considered and possible approaches to avoiding overtreatment are suggested.
Keywords: Canadian National Breast Screening Study; breast cancer; breast cancer screening; microsimulation; overdetection; overdiagnosis; overtreatment.
Conflict of interest statement
Yaffe holds shares in Volpara Health Technologies, a manufacturer of software for analyzing medical images and conducts some collaborative research in breast cancer imaging with GE Healthcare under an agreement with his institution. Neither organization was involved in any way with this project.
Figures




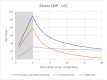

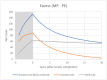
References
-
- Klarenbach S., Sims-Jones N., Lewin G., Singh H., Thériault G., Tonelli M., Doull M., Courage S., Garcia A.J., Thombs B.D. Recommendations on screening for breast cancer in women 40–74 years of age who are not at increased risk for breast cancer. CMAJ. 2018;190:E1441–E1451. doi: 10.1503/cmaj.180463. - DOI - PMC - PubMed
-
- International Agency for Research on Cancer . Breast Cancer Screening IARC Handbook of Cancer Prevention. Volume 15 International Agency for Research on Cancer; Lyon, France: 2016.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

