Optical Properties and Upconversion Luminescence of BaTiO3 Xerogel Structures Doped with Erbium and Ytterbium
- PMID: 35735691
- PMCID: PMC9222966
- DOI: 10.3390/gels8060347
Optical Properties and Upconversion Luminescence of BaTiO3 Xerogel Structures Doped with Erbium and Ytterbium
Abstract
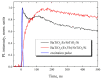
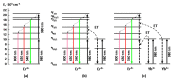
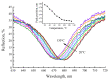
Erbium upconversion (UC) photoluminescence (PL) from sol-gel derived barium titanate (BaTiO3:Er) xerogel structures fabricated on silicon, glass or fused silica substrates has been studied. Under continuous-wave excitation at 980 nm and nanosecond pulsed excitation at 980 and 1540 nm, the fabricated structures demonstrate room temperature PL with several bands at 410, 523, 546, 658, 800 and 830 nm, corresponding to the 2H9/2 → 4I15/2, 2H11/2 → 4I15/2, 4S3/2 → 4I15/2, 4F9/2→ 4I15/2 and 4I9/2→ 4I15/2 transitions of Er3+ ions. The intensity of erbium UC PL increases when an additional macroporous layer of strontium titanate is used beneath the BaTiO3 xerogel layer. It is also enhanced in BaTiO3 xerogel films codoped with erbium and ytterbium (BaTiO3:(Er,Yb)). For the latter, a redistribution of the intensity of the PL bands is observed depending on the excitation conditions. A multilayer BaTiO3:(Er,Yb)/SiO2 microcavity structure was formed on a fused silica substrate with a cavity mode in the range of 650-680 nm corresponding to one of the UC PL bands of Er3+ ions. The obtained cavity structure annealed at 450 °C provides tuning of the cavity mode by 10 nm in the temperature range from 20 °C to 130 °C. Photonic application of BaTiO3 xerogel structures doped with lanthanides is discussed.
Keywords: barium titanate; erbium; luminescence; microcavity; sol-gel; strontium titanate; upconversion; xerogel; ytterbium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Ovsyakin V.V., Feofilov P.P. Cooperative sensitization of luminescence in crystals activated with rare earth ions. JETP Lett. Engl. 1966;4:317–318.
-
- Gibart P., Auzel F., Guillaume J.C., Zahraman K. Below band-gap IR response of substrate-free GaAs solar cells using two-photon up-conversion. Jpn. J. Appl. Phys. 1996;35:4401–4402. doi: 10.1143/JJAP.35.4401. - DOI
-
- Shalav A., Richards B.S., Green M.A. Luminescent layers for enhanced silicon solar cell performance: Up-conversion. Sol. Energy Mater. Sol. Cell. 2007;91:829–842. doi: 10.1016/j.solmat.2007.02.007. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

