Synthesis and Hydrogelation of Star-Shaped Graft Copolypetides with Asymmetric Topology
- PMID: 35735710
- PMCID: PMC9223145
- DOI: 10.3390/gels8060366
Synthesis and Hydrogelation of Star-Shaped Graft Copolypetides with Asymmetric Topology
Abstract
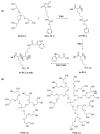
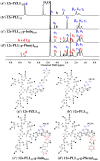
To study the self-assembly and hydrogel formation of the star-shaped graft copolypeptides with asymmetric topology, star-shaped poly(L-lysine) with various arm numbers were synthesized by using asymmetric polyglycerol dendrimers (PGDs) as the initiators and 1,1,3,3-tetramethylguanidine (TMG) as an activator for OH groups, followed by deprotection and grafting with indole or phenyl group on the side chain. The packing of the grafting moiety via non-covalent interactions not only facilitated the polypeptide segments to adopt more ordered conformations but also triggered the spontaneous hydrogelation. The hydrogelation ability was found to be correlated with polypeptide composition and topology. The star-shaped polypeptides with asymmetric topology exhibited poorer hydrogelation ability than those with symmetric topology due to the less efficient packing of the grafted moiety. The star-shaped polypeptides grafted with indole group on the side chain exhibited better hydrogelation ability than those grafted with phenyl group with the same arm number. This report demonstrated that the grafted moiety and polypeptide topology possessed the potential ability to modulate the polypeptide hydrogelation and hydrogel characteristics.
Keywords: hydrogel; polypeptide; star-shaped polymer; topology.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Oyen M. Mechanical characterisation of hydrogel materials. Int. Mater. Rev. 2014;59:44–59. doi: 10.1179/1743280413Y.0000000022. - DOI
-
- Saini K. Preparation method, Properties and Crosslinking of hydrogel: A review. PharmaTutor. 2017;5:27–36.
Grants and funding
- MOST 107-2923-M-006-002-MY3, 108-2221-E-006-034-MY3 and 110-2221-E-006-002-MY3/the Ministry of Science and Technology, Taiwan
- MOST 111-2634-F-006-008/the Hierarchical Green-Energy Materials (Hi-GEM) Research Center, from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) and the Ministry of Science and Technology
LinkOut - more resources
Full Text Sources

