Photoinactivation of Yeast and Biofilm Communities of Candida albicans Mediated by ZnTnHex-2-PyP4+ Porphyrin
- PMID: 35736039
- PMCID: PMC9225021
- DOI: 10.3390/jof8060556
Photoinactivation of Yeast and Biofilm Communities of Candida albicans Mediated by ZnTnHex-2-PyP4+ Porphyrin
Abstract
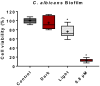
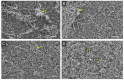
Candida albicans is the main cause of superficial candidiasis. While the antifungals available are defied by biofilm formation and resistance emergence, antimicrobial photodynamic inactivation (aPDI) arises as an alternative antifungal therapy. The tetracationic metalloporphyrin Zn(II) meso-tetrakis(N-n-hexylpyridinium-2-yl)porphyrin (ZnTnHex-2-PyP4+) has high photoefficiency and improved cellular interactions. We investigated the ZnTnHex-2-PyP4+ as a photosensitizer (PS) to photoinactivate yeasts and biofilms of C. albicans strains (ATCC 10231 and ATCC 90028) using a blue light-emitting diode. The photoinactivation of yeasts was evaluated by quantifying the colony forming units. The aPDI of ATCC 90028 biofilms was assessed by the MTT assay, propidium iodide (PI) labeling, and scanning electron microscopy. Mammalian cytotoxicity was investigated in Vero cells using MTT assay. The aPDI (4.3 J/cm2) promoted eradication of yeasts at 0.8 and 1.5 µM of PS for ATCC 10231 and ATCC 90028, respectively. At 0.8 µM and same light dose, aPDI-treated biofilms showed intense PI labeling, about 89% decrease in the cell viability, and structural alterations with reduced hyphae. No considerable toxicity was observed in mammalian cells. Our results introduce the ZnTnHex-2-PyP4+ as a promising PS to photoinactivate both yeasts and biofilms of C. albicans, stimulating studies with other Candida species and resistant isolates.
Keywords: Zn(II) porphyrin; antimicrobial photodynamic inactivation; blue light; fungi; photodynamic therapy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



Similar articles
-
Efficient photodynamic inactivation of Leishmania parasites mediated by lipophilic water-soluble Zn(II) porphyrin ZnTnHex-2-PyP4.Biochim Biophys Acta Gen Subj. 2021 Jul;1865(7):129897. doi: 10.1016/j.bbagen.2021.129897. Epub 2021 Apr 1. Biochim Biophys Acta Gen Subj. 2021. PMID: 33811942
-
A Novel Strategy Based on Zn(II) Porphyrins and Silver Nanoparticles to Photoinactivate Candida albicans.Int J Nanomedicine. 2023 Jun 7;18:3007-3020. doi: 10.2147/IJN.S404422. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37312931 Free PMC article.
-
Fungicidal photodynamic effect of a twofold positively charged porphyrin against Candida albicans planktonic cells and biofilms.Future Microbiol. 2013 Jun;8(6):785-97. doi: 10.2217/fmb.13.44. Future Microbiol. 2013. PMID: 23701333
-
Photodynamic inactivation of Candida albicans by a tetracationic tentacle porphyrin and its analogue without intrinsic charges in presence of fluconazole.Photodiagnosis Photodyn Ther. 2016 Mar;13:334-340. doi: 10.1016/j.pdpdt.2015.10.005. Epub 2015 Oct 21. Photodiagnosis Photodyn Ther. 2016. PMID: 26498876
-
Factors Determining the Susceptibility of Bacteria to Antibacterial Photodynamic Inactivation.Front Med (Lausanne). 2021 May 12;8:642609. doi: 10.3389/fmed.2021.642609. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34055830 Free PMC article. Review.
Cited by
-
The Natural Anthraquinone Parietin Inactivates Candida tropicalis Biofilm by Photodynamic Mechanisms.Pharmaceutics. 2025 Apr 23;17(5):548. doi: 10.3390/pharmaceutics17050548. Pharmaceutics. 2025. PMID: 40430841 Free PMC article.
-
Anticandidal effect of multiple sessions of erythrosine and potassium iodide-mediated photodynamic therapy.J Oral Microbiol. 2024 Jun 18;16(1):2369357. doi: 10.1080/20002297.2024.2369357. eCollection 2024. J Oral Microbiol. 2024. PMID: 38903483 Free PMC article.
-
Photoinactivation of Planktonic Cells, Pseudohyphae, and Biofilms of Candida albicans Sensitized by a Free-Base Chlorin and Its Metal Complexes with Zn(II) and Pd(II).Antibiotics (Basel). 2023 Jan 6;12(1):105. doi: 10.3390/antibiotics12010105. Antibiotics (Basel). 2023. PMID: 36671307 Free PMC article.
-
Antimicrobial Photodynamic Activity of the Zn(II) Phthalocyanine RLP068/Cl Versus Antimicrobial-Resistant Priority Pathogens.Int J Mol Sci. 2025 Aug 5;26(15):7545. doi: 10.3390/ijms26157545. Int J Mol Sci. 2025. PMID: 40806672 Free PMC article.
-
Biofilm: The invisible culprit in catheter-induced candidemia.AIMS Microbiol. 2023 May 11;9(3):467-485. doi: 10.3934/microbiol.2023025. eCollection 2023. AIMS Microbiol. 2023. PMID: 37649801 Free PMC article. Review.
References
Grants and funding
- 219677_Z_19_Z/WT_/Wellcome Trust/United Kingdom
- 406450/2021-8/National Council for Scientific and Technological Development
- APQ-0573-2.09/18/Fundação de Amparo à Ciência e Tecnologia de Pernambuco
- 2018/20226-7/São Paulo Research Foundation
- 465763/2014-6/Instituto Nacional de Ciência e Tecnologia de Fotônica (INCT-INFo)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

