Ethylene Promotes Expression of the Appressorium- and Pathogenicity-Related Genes via GPCR- and MAPK-Dependent Manners in Colletotrichum gloeosporioides
- PMID: 35736053
- PMCID: PMC9224669
- DOI: 10.3390/jof8060570
Ethylene Promotes Expression of the Appressorium- and Pathogenicity-Related Genes via GPCR- and MAPK-Dependent Manners in Colletotrichum gloeosporioides
Abstract
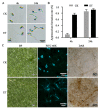
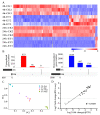
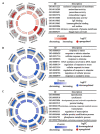
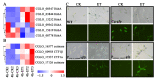
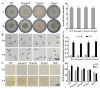
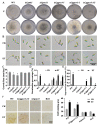
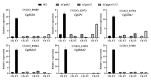
Ethylene (ET) represents a signal that can be sensed by plant pathogenic fungi to accelerate their spore germination and subsequent infection. However, the molecular mechanisms of responses to ET in fungi remain largely unclear. In this study, Colletotrichum gloeosporioides was investigated via transcriptomic analysis to reveal the genes that account for the ET-regulated fungal development and virulence. The results showed that ET promoted genes encoding for fungal melanin biosynthesis enzymes, extracellular hydrolases, and appressorium-associated structure proteins at 4 h after treatment. When the germination lasted until 24 h, ET induced multiple appressoria from every single spore, but downregulated most of the genes. Loss of selected ET responsive genes encoding for scytalone dehydratase (CgSCD1) and cerato-platanin virulence protein (CgCP1) were unable to alter ET sensitivity of C. gloeosporioides in vitro but attenuated the influence of ET on pathogenicity. Knockout of the G-protein-coupled receptors CgGPCR3-1/2 and the MAPK signaling pathway components CgMK1 and CgSte11 resulted in reduced ET sensitivity. Taken together, this study in C. gloeosporioides reports that ET can cause transcription changes in a large set of genes, which are mainly responsible for appressorium development and virulence expression, and these processes are dependent on the GPCR and MAPK pathways.
Keywords: GPCRs; MAPK; anthracnose; appressorium; ethylene; postharvest disease.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

