Fungal-Bacterial Networks in the Habitat of SongRong (Tricholoma matsutake) and Driving Factors of Their Distribution Rules
- PMID: 35736058
- PMCID: PMC9225054
- DOI: 10.3390/jof8060575
Fungal-Bacterial Networks in the Habitat of SongRong (Tricholoma matsutake) and Driving Factors of Their Distribution Rules
Abstract
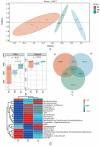
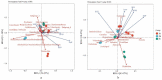
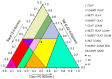
Soil origin, mycorrhizal plant partners and environmental factors affect the growth and development of SongRong (Tricholoma matsutake). In order to clarify the relationships of fungi-bacteria networks and various influence factors in the habitat of SongRong, we chose three collection sites with a Quercus mongolica pure forest (plot A without SongRong was used as the control sample site), Q. mongolica mixed Rhododendron dauricum (plot B) and Q. mongolica mixed with R. dauricum and Pinus densiflora (plot C). By using high-throughput sequencing, we obtained a total of 4930 fungal and 55501 bacterial amplicon sequence variants (ASVs) based on internally transcribed spacer ribosomal RNA (ITS rRNA) and 16S ribosomal RNA (16S rRNA) sequencing via the Illumina NovaSeq platform. In the habitat soil of SongRong (plot B and plot C), alpha or beta diversity and species compositions of fungi and bacteria were different from plot A. The fungal-bacterial networks follow the selection rule that few dominant genera account for the greater relative abundance. Forest types, but not the host itself, drove the fungal-bacterial networks of the forest soil, and soil physicochemical characteristics and texture affected their abundance. The abundance of Tricholoma was affected by the fungal and bacterial abundance in the habitat.
Keywords: Tricholoma matsutake; forestry types; fungi–bacterial networks; mixed forest with Quercus mongolica; soil physicochemical parameters; soil structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The Diverse Mycorrizal Morphology of Rhododendron dauricum, the Fungal Communities Structure and Dynamics from the Mycorrhizosphere.J Fungi (Basel). 2024 Jan 14;10(1):65. doi: 10.3390/jof10010065. J Fungi (Basel). 2024. PMID: 38248974 Free PMC article.
-
Ectomycorrhization of Tricholoma matsutake with Quercus aquifolioides affects the endophytic microbial community of host plant.J Basic Microbiol. 2018 Mar;58(3):238-246. doi: 10.1002/jobm.201700506. Epub 2018 Jan 23. J Basic Microbiol. 2018. PMID: 29359810
-
Fungal Interactions Matter: Tricholoma matsutake Domination Affect Fungal Diversity and Function in Mountain Forest Soils.Biology (Basel). 2021 Oct 15;10(10):1051. doi: 10.3390/biology10101051. Biology (Basel). 2021. PMID: 34681150 Free PMC article.
-
Pyrosequencing and Taxonomic Composition of the Fungal Community from Soil of Tricholoma matsutake in Gyeongju.J Microbiol Biotechnol. 2021 May 28;31(5):686-695. doi: 10.4014/jmb.2103.03021. J Microbiol Biotechnol. 2021. PMID: 33782219 Free PMC article.
-
Root-associated bacteria influencing mycelial growth of Tricholoma matsutake (pine mushroom).J Microbiol. 2018 Jun;56(6):399-407. doi: 10.1007/s12275-018-7491-y. Epub 2018 Jun 1. J Microbiol. 2018. PMID: 29858828
Cited by
-
Edible and Medicinal Macrofungi.J Fungi (Basel). 2023 Sep 7;9(9):908. doi: 10.3390/jof9090908. J Fungi (Basel). 2023. PMID: 37755016 Free PMC article.
-
The regulation of tobacco growth under preceding crop planting: insights from soil quality, microbial communities, and metabolic profiling.Front Plant Sci. 2025 Feb 7;16:1530324. doi: 10.3389/fpls.2025.1530324. eCollection 2025. Front Plant Sci. 2025. PMID: 39990714 Free PMC article.
-
Soil microbiome of shiro reveals the symbiotic relationship between Tricholoma bakamatsutake and Quercus mongolica.Front Microbiol. 2024 Mar 27;15:1361117. doi: 10.3389/fmicb.2024.1361117. eCollection 2024. Front Microbiol. 2024. PMID: 38601932 Free PMC article.
-
The Diverse Mycorrizal Morphology of Rhododendron dauricum, the Fungal Communities Structure and Dynamics from the Mycorrhizosphere.J Fungi (Basel). 2024 Jan 14;10(1):65. doi: 10.3390/jof10010065. J Fungi (Basel). 2024. PMID: 38248974 Free PMC article.
References
-
- Wang Y., Yu F.Q., Zhang C.X., Li S.H. Tricholoma matsutake: An edible mycorrhizal mushroom of high socioeconomic relevance in China. Rev. Mex. Micol. 2017;46:55–61.
-
- Amend A., Garbelotto M., Fang Z.D., Keeley S. Isolation by landscape in populations of a prized edible mushroom Tricholoma matsutake. Conserv. Genet. 2010;11:795–802. doi: 10.1007/s10592-009-9894-0. - DOI
-
- Yao Y.J., Wei J.C., Zhuang W.Y., Cai L., Liu D.M., Li J.S., Li Y., Wang K., Wu H.J. Development of red list assessment of macrofungi in China. Sheng Wu Duo Yang Xing. 2020;28:4. doi: 10.17520/biods.2019173. - DOI
-
- Xian M.Y. Distribution and ecological environment of Tricholoma matsutake in Sichuan Province. Edible Fungi. 1989;5:9–10.
-
- Liao S.Y., Leng H.Q., Liu B. The special ecological environment of matsutake mushroom in Sichuan. J. Sichuan Agric. Univ. 1991;2:297–302.
Grants and funding
LinkOut - more resources
Full Text Sources

