Purification and Characterization of a Novel α-L-Rhamnosidase from Papiliotrema laurentii ZJU-L07 and Its Application in Production of Icariin from Epimedin C
- PMID: 35736128
- PMCID: PMC9225045
- DOI: 10.3390/jof8060644
Purification and Characterization of a Novel α-L-Rhamnosidase from Papiliotrema laurentii ZJU-L07 and Its Application in Production of Icariin from Epimedin C
Abstract
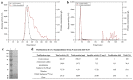
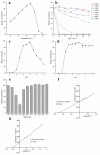
Icariin is the most effective bioactive compound in Herba Epimedii. To enhance the content of icariin in the epimedium water extract, a novel strain, Papiliotrema laurentii ZJU-L07, producing an intracellular α-L-rhamnosidase was isolated from the soil and mutagenized. The specific activity of α-L-rhamnosidase was 29.89 U·mg-1 through purification, and the molecular mass of the enzyme was 100 kDa, as assayed by SDS-PAGE. The characterization of the purified enzyme was determined. The optimal temperature and pH were 55 °C and 7.0, respectively. The enzyme was stable in the pH range 5.5-9.0 for 2 h over 80% and the temperature range 30-40 °C for 2 h more than 70%. The enzyme activity was inhibited by Ca2+, Fe2+, Cu2+, and Mg2+, especially Fe2+. The kinetic parameters of Km and Vmax were 1.38 mM and 24.64 μmol·mg-1·min-1 using pNPR as the substrate, respectively. When epimedin C was used as a nature substrate to determine the kinetic parameters of α-L-rhamnosidase, the values of Km and Vmax were 3.28 mM and 0.01 μmol·mg-1·min-1, respectively. The conditions of enzymatic hydrolysis were optimized through single factor experiments and response surface methodology. The icariin yield increased from 61% to over 83% after optimization. The enzymatic hydrolysis method could be used for the industrialized production of icariin. At the same time, this enzyme could also cleave the α-1,2 glycosidic linkage between glucoside and rhamnoside in naringin and neohesperidin, which could be applicable in other biotechnological processes.
Keywords: Papiliotrema laurentii ZJU-L07; enzyme characteristics; epimedin C; icariin; optimization; α-L-rhamnosidase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
An Organic Solvent-Tolerant α-L-Rhamnosidase from Dictyoglomus thermophilum and Its Application in Production of Icariside I from Icariin.Molecules. 2025 Jul 3;30(13):2847. doi: 10.3390/molecules30132847. Molecules. 2025. PMID: 40649361 Free PMC article.
-
Characterization of a α-l-rhamnosidase from Bacteroides thetaiotaomicron with high catalytic efficiency of epimedin C.Bioorg Chem. 2018 Dec;81:461-467. doi: 10.1016/j.bioorg.2018.08.004. Epub 2018 Sep 6. Bioorg Chem. 2018. PMID: 30243237
-
Screening and characterization of a β-xylosidase from Bifidobacterium breve K-110 and its application in the biotransformation of the total flavonoids of epimedium to icariin with α-l-rhamnosidase.Bioorg Chem. 2023 Mar;132:106364. doi: 10.1016/j.bioorg.2023.106364. Epub 2023 Jan 16. Bioorg Chem. 2023. PMID: 36706530
-
Efficient bioconversion of epimedin C to icariin by a glycosidase from Aspergillus nidulans.Bioresour Technol. 2019 Oct;289:121612. doi: 10.1016/j.biortech.2019.121612. Epub 2019 Jun 6. Bioresour Technol. 2019. PMID: 31203178
-
[Enzymatic properties of α-L-rhamnosidase and the factors affecting its activity: a review].Sheng Wu Gong Cheng Xue Bao. 2021 Aug 25;37(8):2623-2632. doi: 10.13345/j.cjb.200565. Sheng Wu Gong Cheng Xue Bao. 2021. PMID: 34472283 Review. Chinese.
Cited by
-
An Organic Solvent-Tolerant α-L-Rhamnosidase from Dictyoglomus thermophilum and Its Application in Production of Icariside I from Icariin.Molecules. 2025 Jul 3;30(13):2847. doi: 10.3390/molecules30132847. Molecules. 2025. PMID: 40649361 Free PMC article.
-
Heterologous Expression and Characterization of a Thermostable α-L-Rhamnosidase from Thermoclostridium stercorarium subsp. thermolacticum DSM 2910 and Its Application in the Biotransformation of Rutin.J Microbiol Biotechnol. 2023 Nov 28;33(11):1521-1530. doi: 10.4014/jmb.2305.05032. Epub 2023 Jul 21. J Microbiol Biotechnol. 2023. PMID: 37644729 Free PMC article.
-
Advancements in the Biotransformation and Biosynthesis of the Primary Active Flavonoids Derived from Epimedium.Molecules. 2023 Oct 19;28(20):7173. doi: 10.3390/molecules28207173. Molecules. 2023. PMID: 37894651 Free PMC article. Review.
References
-
- Jiang J., Song J., Jia X.-B. Phytochemistry and Ethnopharmacology of Epimedium L. Species. Chin. Herb. Med. 2015;7:204–222. doi: 10.1016/S1674-6384(15)60043-0. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

