Oncolytic Vaccinia Virus Harboring Aphrocallistes vastus Lectin Inhibits the Growth of Hepatocellular Carcinoma Cells
- PMID: 35736181
- PMCID: PMC9230886
- DOI: 10.3390/md20060378
Oncolytic Vaccinia Virus Harboring Aphrocallistes vastus Lectin Inhibits the Growth of Hepatocellular Carcinoma Cells
Abstract
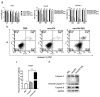
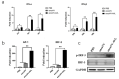
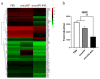
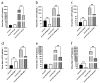
Oncolytic vaccinia virus has been developed as a novel cancer therapeutic drug in recent years. Our previous studies demonstrated that the antitumor effect of oncolytic vaccina virus harboring Aphrocallistes vastus lectin (oncoVV-AVL) was significantly enhanced in several cancer cells. In the present study, we investigated the underlying mechanisms of AVL that affect virus replication and promote the antitumor efficacy of oncolytic virus in hepatocellular carcinoma (HCC). Our results showed that oncoVV-AVL markedly exhibited antitumor effects in both hepatocellular carcinoma cell lines and a xenograft mouse model. Further investigation illustrated that oncoVV-AVL could activate tumor immunity by upregulating the expression of type I interferons and enhance virus replication by inhibiting ISRE mediated viral defense response. In addition, we inferred that AVL promoted the ability of virus replication by regulating the PI3K/Akt, MAPK/ERK, and Hippo/MST pathways through cross-talk Raf-1, as well as metabolism-related pathways. These findings provide a novel perspective for the exploitation of marine lectins in oncolytic therapy.
Keywords: Aphrocallistes vastus lectin; Huh7 cells; oncolytic vaccinia virus; viral replication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Oncolytic Vaccinia Virus Carrying Aphrocallistes vastus Lectin (oncoVV-AVL) Enhances Inflammatory Response in Hepatocellular Carcinoma Cells.Mar Drugs. 2022 Oct 26;20(11):667. doi: 10.3390/md20110667. Mar Drugs. 2022. PMID: 36354990 Free PMC article.
-
Effects of Oncolytic Vaccinia Viruses Harboring Different Marine Lectins on Hepatocellular Carcinoma Cells.Int J Mol Sci. 2023 Feb 14;24(4):3823. doi: 10.3390/ijms24043823. Int J Mol Sci. 2023. PMID: 36835232 Free PMC article.
-
Oncolytic Vaccinia Virus Expressing Aphrocallistes vastus Lectin as a Cancer Therapeutic Agent.Mar Drugs. 2019 Jun 19;17(6):363. doi: 10.3390/md17060363. Mar Drugs. 2019. PMID: 31248066 Free PMC article.
-
Vaccinia virus, a promising new therapeutic agent for pancreatic cancer.Immunotherapy. 2015;7(12):1249-58. doi: 10.2217/imt.15.90. Epub 2015 Nov 23. Immunotherapy. 2015. PMID: 26595180 Free PMC article. Review.
-
JX-594, a targeted oncolytic poxvirus for the treatment of cancer.Curr Opin Investig Drugs. 2009 Dec;10(12):1372-82. Curr Opin Investig Drugs. 2009. PMID: 19943208 Review.
Cited by
-
ROS Induced by Aphrocallistes vastus Lectin Enhance Oncolytic Vaccinia Virus Replication and Induce Apoptosis in Hepatocellular Carcinoma Cells.Mar Drugs. 2024 Jun 30;22(7):307. doi: 10.3390/md22070307. Mar Drugs. 2024. PMID: 39057416 Free PMC article.
-
A Comparative Study of Oncolytic Vaccinia Viruses Harboring Different Marine Lectins in Breast Cancer Cells.Mar Drugs. 2023 Jan 23;21(2):77. doi: 10.3390/md21020077. Mar Drugs. 2023. PMID: 36827118 Free PMC article.
-
Oncolytic vaccinia virus harboring CLEC2A gene enhances viral replication and antitumor efficacy.Mol Ther Oncol. 2024 Jun 5;32(3):200823. doi: 10.1016/j.omton.2024.200823. eCollection 2024 Sep 19. Mol Ther Oncol. 2024. PMID: 39006946 Free PMC article.
-
Progression of oncolytic virus in liver cancer treatment.Front Oncol. 2024 Sep 26;14:1446085. doi: 10.3389/fonc.2024.1446085. eCollection 2024. Front Oncol. 2024. PMID: 39391253 Free PMC article. Review.
-
Oncolytic Vaccinia Virus Carrying Aphrocallistes vastus Lectin (oncoVV-AVL) Enhances Inflammatory Response in Hepatocellular Carcinoma Cells.Mar Drugs. 2022 Oct 26;20(11):667. doi: 10.3390/md20110667. Mar Drugs. 2022. PMID: 36354990 Free PMC article.
References
-
- Wang J.H., Arulanandam R., Wassenaar R., Falls T., Petryk J., Paget J., Garson K., Cemeus C., Vanderhyden B.C., Wells R.G., et al. Enhancing expression of functional human sodium Iodide symporter and somatostatin receptor in recombinant oncolytic vaccinia virus for in vivo imaging of tumors. J. Nucl. Med. 2017;58:221–227. doi: 10.2967/jnumed.116.180463. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

