Anatomy of four human Argonaute proteins
- PMID: 35736234
- PMCID: PMC9262622
- DOI: 10.1093/nar/gkac519
Anatomy of four human Argonaute proteins
Abstract
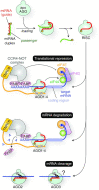
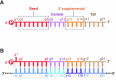
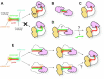
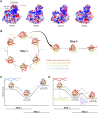
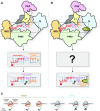
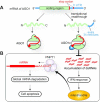
MicroRNAs (miRNAs) bind to complementary target RNAs and regulate their gene expression post-transcriptionally. These non-coding regulatory RNAs become functional after loading into Argonaute (AGO) proteins to form the effector complexes. Humans have four AGO proteins, AGO1, AGO2, AGO3 and AGO4, which share a high sequence identity. Since most miRNAs are found across the four AGOs, it has been thought that they work redundantly, and AGO2 has been heavily studied as the exemplified human paralog. Nevertheless, an increasing number of studies have found that the other paralogs play unique roles in various biological processes and diseases. In the last decade, the structural study of the four AGOs has provided the field with solid structural bases. This review exploits the completed structural catalog to describe common features and differences in target specificity across the four AGOs.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


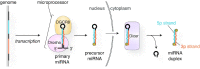
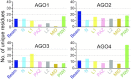
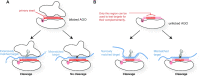
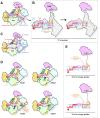

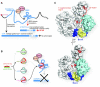



References
-
- Sasaki T., Shiohama A., Minoshima S., Shimizu N.. Identification of eight members of the argonaute family in the human genome. Genomics. 2003; 82:323–330. - PubMed
-
- Fagerberg L., Hallstrom B.M., Oksvold P., Kampf C., Djureinovic D., Odeberg J., Habuka M., Tahmasebpoor S., Danielsson A., Edlund K.et al. .. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell Proteomics. 2014; 13:397–406. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

