Importance of Hydroxide Ion Conductivity Measurement for Alkaline Water Electrolysis Membranes
- PMID: 35736263
- PMCID: PMC9229372
- DOI: 10.3390/membranes12060556
Importance of Hydroxide Ion Conductivity Measurement for Alkaline Water Electrolysis Membranes
Abstract
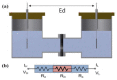
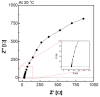
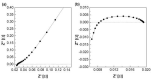
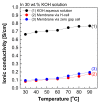
Alkaline water electrolysis (AWE) refers to a representative water electrolysis technology that applies electricity to synthesize hydrogen gas without the production of carbon dioxide. The ideal polymer electrolyte membranes for AWE should be capable of transporting hydroxide ions (OH-) quickly in harsh alkaline environments at increased temperatures. However, there has not yet been any desirable impedance measurement method for estimating hydroxide ions' conduction behavior across the membranes, since their impedance spectra are significantly affected by connection modes between electrodes and membranes in the test cells and the impedance evaluation environments. Accordingly, the measurement method suitable for obtaining precise hydroxide ion conductivity values through the membranes should be determined. For this purpose, Zirfon®, a state-of-the-art AWE membrane, was adopted as the standard membrane sample to perform the impedance measurement. The impedance spectra were acquired using homemade test cells with different electrode configurations in alkaline environments, and the corresponding hydroxide ion conductivity values were determined based on the electrochemical spectra. Furthermore, a modified four-probe method was found as an optimal measurement method by comparing the conductivity obtained under alkaline conditions.
Keywords: Zirfon; alkaline water electrolysis; electrode configuration; hydrogen generation; hydroxide ion transport; impedance measurement; ion conductivity; polymer electrolyte membrane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Santos D.M., Sequeira C.A., Figueiredo J.L.J.Q.N. Hydrogen production by alkaline water electrolysis. Química Nova. 2013;36:1176–1193. doi: 10.1590/S0100-40422013000800017. - DOI
-
- Shiva Kumar S., Himabindu V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019;2:442–454. doi: 10.1016/j.mset.2019.03.002. - DOI
-
- Zeng K., Zhang D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010;36:307–326. doi: 10.1016/j.pecs.2009.11.002. - DOI
-
- Varcoe J.R., Atanassov P., Dekel D.R., Herring A.M., Hickner M.A., Kohl P.A., Kucernak A.R., Mustain W.E., Nijmeijer K., Scott K., et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 2014;7:3135–3191. doi: 10.1039/C4EE01303D. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

