G1-Cyclin2 (Cln2) promotes chromosome hypercondensation in eco1/ctf7 rad61 null cells during hyperthermic stress in Saccharomyces cerevisiae
- PMID: 35736360
- PMCID: PMC9339302
- DOI: 10.1093/g3journal/jkac157
G1-Cyclin2 (Cln2) promotes chromosome hypercondensation in eco1/ctf7 rad61 null cells during hyperthermic stress in Saccharomyces cerevisiae
Abstract
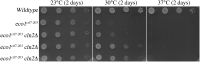
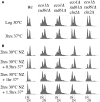
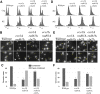
Eco1/Ctf7 is a highly conserved acetyltransferase that activates cohesin complexes and is critical for sister chromatid cohesion, chromosome condensation, DNA damage repair, nucleolar integrity, and gene transcription. Mutations in the human homolog of ECO1 (ESCO2/EFO2), or in genes that encode cohesin subunits, result in severe developmental abnormalities and intellectual disabilities referred to as Roberts syndrome and Cornelia de Lange syndrome, respectively. In yeast, deletion of ECO1 results in cell inviability. Codeletion of RAD61 (WAPL in humans), however, produces viable yeast cells. These eco1 rad61 double mutants, however, exhibit a severe temperature-sensitive growth defect, suggesting that Eco1 or cohesins respond to hyperthermic stress through a mechanism that occurs independent of Rad61. Here, we report that deletion of the G1 cyclin CLN2 rescues the temperature-sensitive lethality otherwise exhibited by eco1 rad61 mutant cells, such that the triple mutant cells exhibit robust growth over a broad range of temperatures. While Cln1, Cln2, and Cln3 are functionally redundant G1 cyclins, neither CLN1 nor CLN3 deletions rescue the temperature-sensitive growth defects otherwise exhibited by eco1 rad61 double mutants. We further provide evidence that CLN2 deletion rescues hyperthermic growth defects independent of START and impacts the state of chromosome condensation. These findings reveal novel roles for Cln2 that are unique among the G1 cyclin family and appear critical for cohesin regulation during hyperthermic stress.
Keywords: Cornelia de Lange syndrome (CdLS); Eco1/ESCO2; G1 Cyclin/Cln2; Roberts syndrome (RBS); chromosome condensation; cohesinopathies; sister chromatid cohesion.
© The Author(s) 2022. Published by Oxford University Press on behalf of Genetics Society of America.
Figures




Similar articles
-
Fdo1, Fkh1, Fkh2, and the Swi6-Mbp1 MBF complex regulate Mcd1 levels to impact eco1 rad61 cell growth in Saccharomyces cerevisiae.Genetics. 2024 Oct 7;228(2):iyae128. doi: 10.1093/genetics/iyae128. Genetics. 2024. PMID: 39110836 Free PMC article.
-
Analysis of combinatorial cohesin subunit gene deletions in budding yeast.Genetics. 2025 Aug 6;230(4):iyaf107. doi: 10.1093/genetics/iyaf107. Genetics. 2025. PMID: 40492837
-
The acetyltransferase Eco1 elicits cohesin dimerization during S phase.J Biol Chem. 2020 May 29;295(22):7554-7565. doi: 10.1074/jbc.RA120.013102. Epub 2020 Apr 20. J Biol Chem. 2020. PMID: 32312753 Free PMC article.
-
The expanding phenotypes of cohesinopathies: one ring to rule them all!Cell Cycle. 2019 Nov;18(21):2828-2848. doi: 10.1080/15384101.2019.1658476. Epub 2019 Sep 13. Cell Cycle. 2019. PMID: 31516082 Free PMC article. Review.
-
Integrating Sister Chromatid Cohesion Establishment to DNA Replication.Genes (Basel). 2022 Mar 31;13(4):625. doi: 10.3390/genes13040625. Genes (Basel). 2022. PMID: 35456431 Free PMC article. Review.
Cited by
-
Fdo1, Fkh1, Fkh2, and the Swi6-Mbp1 MBF complex regulate Mcd1 levels to impact eco1 rad61 cell growth in Saccharomyces cerevisiae.Genetics. 2024 Oct 7;228(2):iyae128. doi: 10.1093/genetics/iyae128. Genetics. 2024. PMID: 39110836 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
