Nutritional Interactions between Bacterial Species Colonising the Human Nasal Cavity: Current Knowledge and Future Prospects
- PMID: 35736422
- PMCID: PMC9229137
- DOI: 10.3390/metabo12060489
Nutritional Interactions between Bacterial Species Colonising the Human Nasal Cavity: Current Knowledge and Future Prospects
Abstract
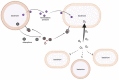
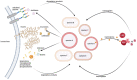
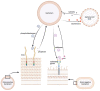
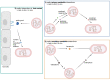
The human nasal microbiome can be a reservoir for several pathogens, including Staphylococcus aureus. However, certain harmless nasal commensals can interfere with pathogen colonisation, an ability that could be exploited to prevent infection. Although attractive as a prophylactic strategy, manipulation of nasal microbiomes to prevent pathogen colonisation requires a better understanding of the molecular mechanisms of interaction that occur between nasal commensals as well as between commensals and pathogens. Our knowledge concerning the mechanisms of pathogen exclusion and how stable community structures are established is patchy and incomplete. Nutrients are scarce in nasal cavities, which makes competitive or mutualistic traits in nutrient acquisition very likely. In this review, we focus on nutritional interactions that have been shown to or might occur between nasal microbiome members. We summarise concepts of nutrient release from complex host molecules and host cells as well as of intracommunity exchange of energy-rich fermentation products and siderophores. Finally, we discuss the potential of genome-based metabolic models to predict complex nutritional interactions between members of the nasal microbiome.
Keywords: Staphylococcus aureus; bacterial interaction; nasal microbiome; nutritional interaction.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Competition among Nasal Bacteria Suggests a Role for Siderophore-Mediated Interactions in Shaping the Human Nasal Microbiota.Appl Environ Microbiol. 2019 May 2;85(10):e02406-18. doi: 10.1128/AEM.02406-18. Print 2019 May 15. Appl Environ Microbiol. 2019. PMID: 30578265 Free PMC article.
-
Identification of Nasal Gammaproteobacteria with Potent Activity against Staphylococcus aureus: Novel Insights into the "Noncarrier" State.mSphere. 2021 Jan 6;6(1):e01015-20. doi: 10.1128/mSphere.01015-20. mSphere. 2021. PMID: 33408227 Free PMC article.
-
Staphylococcus aureus and the ecology of the nasal microbiome.Sci Adv. 2015 Jun 5;1(5):e1400216. doi: 10.1126/sciadv.1400216. eCollection 2015 Jun. Sci Adv. 2015. PMID: 26601194 Free PMC article.
-
Secondary Metabolites Governing Microbiome Interaction of Staphylococcal Pathogens and Commensals.Microb Physiol. 2021;31(3):198-216. doi: 10.1159/000517082. Epub 2021 Jul 29. Microb Physiol. 2021. PMID: 34325424 Review.
-
Nasal Microbiome and Its Interaction with the Host in Childhood Asthma.Cells. 2022 Oct 7;11(19):3155. doi: 10.3390/cells11193155. Cells. 2022. PMID: 36231116 Free PMC article. Review.
Cited by
-
Metabolic capabilities are highly conserved among human nasal-associated Corynebacterium species in pangenomic analyses.mSystems. 2024 Dec 17;9(12):e0113224. doi: 10.1128/msystems.01132-24. Epub 2024 Nov 7. mSystems. 2024. PMID: 39508593 Free PMC article.
-
Temperature influences commensal-pathogen dynamics in a nasal epithelial cell co-culture model.mSphere. 2024 Jan 30;9(1):e0058923. doi: 10.1128/msphere.00589-23. Epub 2024 Jan 5. mSphere. 2024. PMID: 38179905 Free PMC article.
-
A Perioperative Disinfection and Caring Procedure to Prevent Infection After Rhinoplasty.Aesthetic Plast Surg. 2023 Oct;47(5):2217-2218. doi: 10.1007/s00266-023-03414-y. Epub 2023 Jun 7. Aesthetic Plast Surg. 2023. PMID: 37286843 No abstract available.
-
Nasal commensals reduce Staphylococcus aureus proliferation by restricting siderophore availability.ISME J. 2024 Jan 8;18(1):wrae123. doi: 10.1093/ismejo/wrae123. ISME J. 2024. PMID: 38987933 Free PMC article.
-
In vitro metabolic interaction network of a rationally designed nasal microbiota community.iScience. 2025 Jul 14;28(8):113114. doi: 10.1016/j.isci.2025.113114. eCollection 2025 Aug 15. iScience. 2025. PMID: 40761294 Free PMC article.
References
-
- Bode L.G., Kluytmans J.A., Wertheim H.F., Bogaers D., Vandenbroucke-Grauls C.M., Roosendaal R., Troelstra A., Box A.T., Voss A., van der Tweel I., et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N. Engl. J. Med. 2010;362:9–17. doi: 10.1056/NEJMoa0808939. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

