Metabolic Determinants in Cardiomyocyte Function and Heart Regenerative Strategies
- PMID: 35736435
- PMCID: PMC9227827
- DOI: 10.3390/metabo12060500
Metabolic Determinants in Cardiomyocyte Function and Heart Regenerative Strategies
Abstract
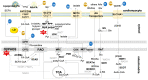
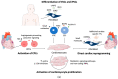
Heart disease is the leading cause of mortality in developed countries. The associated pathology is characterized by a loss of cardiomyocytes that leads, eventually, to heart failure. In this context, several cardiac regenerative strategies have been developed, but they still lack clinical effectiveness. The mammalian neonatal heart is capable of substantial regeneration following injury, but this capacity is lost at postnatal stages when cardiomyocytes become terminally differentiated and transit to the fetal metabolic switch. Cardiomyocytes are metabolically versatile cells capable of using an array of fuel sources, and the metabolism of cardiomyocytes suffers extended reprogramming after injury. Apart from energetic sources, metabolites are emerging regulators of epigenetic programs driving cell pluripotency and differentiation. Thus, understanding the metabolic determinants that regulate cardiomyocyte maturation and function is key for unlocking future metabolic interventions for cardiac regeneration. In this review, we will discuss the emerging role of metabolism and nutrient signaling in cardiomyocyte function and repair, as well as whether exploiting this axis could potentiate current cellular regenerative strategies for the mammalian heart.
Keywords: cardiac regeneration; cardiomyocytes; cell reprogramming; metabolism; mitochondria; nutrient signaling; pluripotency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Neidig L.E., Weinberger F., Palpant N.J., Mignone J., Martinson A.M., Sorensen D.W., Bender I., Nemoto N., Reinecke H., Pabon L., et al. Evidence for minimal cardiogenic potential of stem cell antigen 1-positive cells in the adult mouse heart. Circulation. 2018;138:2960–2962. doi: 10.1161/CIRCULATIONAHA.118.035273. - DOI - PMC - PubMed
Publication types
Grants and funding
- ERA-CVD/0001/3599-PPCDT/2018-INNOVATION/Fundação para a Ciência e Tecnologia
- EXPL/BIA-CEL/0358/2021/Fundação para a Ciência e Tecnologia
- UI/BD/151373/2021/Fundação para a Ciência e Tecnologia
- SFRH/BD/146204/2019/Fundação para a Ciência e Tecnologia
- LISBOA-01-0145-FEDER-028534/projeto cofinanciado pelo FEDER através POR Lisboa 2020 - Programa Operacional Regional de Lisboa, do PORTUGAL 2020 e pela Fundação para a Ciência e a Tecnologia
LinkOut - more resources
Full Text Sources

