A Quantitative Systems Pharmacology Platform Reveals NAFLD Pathophysiological States and Targeting Strategies
- PMID: 35736460
- PMCID: PMC9227696
- DOI: 10.3390/metabo12060528
A Quantitative Systems Pharmacology Platform Reveals NAFLD Pathophysiological States and Targeting Strategies
Abstract
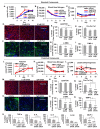
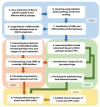
Non-alcoholic fatty liver disease (NAFLD) has a high global prevalence with a heterogeneous and complex pathophysiology that presents barriers to traditional targeted therapeutic approaches. We describe an integrated quantitative systems pharmacology (QSP) platform that comprehensively and unbiasedly defines disease states, in contrast to just individual genes or pathways, that promote NAFLD progression. The QSP platform can be used to predict drugs that normalize these disease states and experimentally test predictions in a human liver acinus microphysiology system (LAMPS) that recapitulates key aspects of NAFLD. Analysis of a 182 patient-derived hepatic RNA-sequencing dataset generated 12 gene signatures mirroring these states. Screening against the LINCS L1000 database led to the identification of drugs predicted to revert these signatures and corresponding disease states. A proof-of-concept study in LAMPS demonstrated mitigation of steatosis, inflammation, and fibrosis, especially with drug combinations. Mechanistically, several structurally diverse drugs were predicted to interact with a subnetwork of nuclear receptors, including pregnane X receptor (PXR; NR1I2), that has evolved to respond to both xenobiotic and endogenous ligands and is intrinsic to NAFLD-associated transcription dysregulation. In conjunction with iPSC-derived cells, this platform has the potential for developing personalized NAFLD therapeutic strategies, informing disease mechanisms, and defining optimal cohorts of patients for clinical trials.
Keywords: CMap; MAFLD; MPS; NAFLD; NASH; QSP; connectivity map; drug combinations; drug discovery; drug repurposing; fibrosis; liver; lobular inflammation; metabolic-associated fatty liver disease; microphysiology systems; network proximity; non-alcoholic fatty liver disease; non-alcoholic steatohepatitis; quantitative systems pharmacology; steatosis; targeting disease states.
Conflict of interest statement
D.L.T., A.G. and L.A.V. are the co-inventors and owners (TaylorGoughVernetti, LLP) of a patent on the construction and use of the biomimetic liver MPS. D.L.T. and A.G. are cofounders of BioSystics, Inc., a company developing patient digital twins starting with accessing, analyzing, and computationally modeling data on patient-derived microphysiology systems. Their interests are managed by the Conflict-of-Interest Office at the University of Pittsburgh, USA, in accordance with their policies. A.S.-G. is a co-founder, and D.L.T. is an advisor for Von Baer Wolff, Inc., a company focused on biofabrication of autologous human hepatocytes using stem cell technology. A.S.-G. is a co-founder of and owns stock in Pittsburgh ReLiver Inc., a company focused on genetic reprogramming to overcome liver failure. He is a coinventor on a patent application that describes the use of transcription factors to treat chronic liver failure and on a patent application related to methods to enhance hepatic functions in failing human livers. The other authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures




References
Grants and funding
- S10 OD028540/OD/NIH HHS/United States
- U01 TR002383/TR/NCATS NIH HHS/United States
- P30 CA047904/CA/NCI NIH HHS/United States
- UH3 DK119973/DK/NIDDK NIH HHS/United States
- P01 DK096990/DK/NIDDK NIH HHS/United States
- P41 GM103712/GM/NIGMS NIH HHS/United States
- S10OD12269-NIH/NH/NIH HHS/United States
- R01 DK088231-NIH/NIDDK/NH/NIH HHS/United States
- U24 TR002632/TR/NCATS NIH HHS/United States
- UG3 TR003289/TR/NCATS NIH HHS/United States
- P41 GM103712 NIH/NIGMS/NH/NIH HHS/United States
- R01 DK097160-NIH/NIDDK/NH/NIH HHS/United States
- R01 DK097160/DK/NIDDK NIH HHS/United States
- U24TR002632-NIH/NCATS/NH/NIH HHS/United States
- PO1 DK096990 NIH/NIDDK/NH/NIH HHS/United States
- R01 CA255809/CA/NCI NIH HHS/United States
- 1UG3TR003289-01-NIH/NCATS/NH/NIH HHS/United States
- R01 GM139297/GM/NIGMS NIH HHS/United States
- R01 DK062277/DK/NIDDK NIH HHS/United States
- R01 DK088231/DK/NIDDK NIH HHS/United States
- 1P30DK120531-01/NH/NIH HHS/United States
- S10 OD012269/OD/NIH HHS/United States
- P30 DK120531/DK/NIDDK NIH HHS/United States
- R01 DK117881/DK/NIDDK NIH HHS/United States
- 5U01 TR002383-03-NIH/NCATS/NH/NIH HHS/United States
- S10 OD028450/OD/NIH HHS/United States
- UH3 TR003289/TR/NCATS NIH HHS/United States
- 4UH3DK119973-03-NIH/NIDDK/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

