CRISPR/CasRx-Mediated RNA Knockdown Reveals That ACE2 Is Involved in the Regulation of Oligodendroglial Cell Morphological Differentiation
- PMID: 35736639
- PMCID: PMC9229887
- DOI: 10.3390/ncrna8030042
CRISPR/CasRx-Mediated RNA Knockdown Reveals That ACE2 Is Involved in the Regulation of Oligodendroglial Cell Morphological Differentiation
Abstract
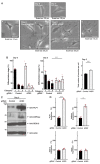
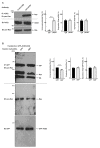
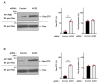
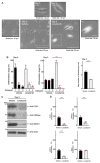
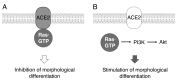
Angiotensin-converting enzyme 2 (ACE2) plays a role in catalyzing angiotensin II conversion to angiotensin (1-7), which often counteracts the renin-angiotensin system. ACE2 is expressed not only in the cells of peripheral tissues such as the heart and kidney, but also in those of the central nervous system (CNS). Additionally, ACE2 acts as the receptor required for the entry of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), whose binding leads to endocytotic recycling and possible degradation of the ACE2 proteins themselves. One of the target cells for SARS-CoV-2 in the CNS is oligodendrocytes (oligodendroglial cells), which wrap neuronal axons with their differentiated plasma membranes called myelin membranes. Here, for the first time, we describe the role of ACE2 in FBD-102b cells, which are used as the differentiation models of oligodendroglial cells. Unexpectedly, RNA knockdown of ACE2 with CasRx-mediated gRNA or the cognate siRNA promoted oligodendroglial cell morphological differentiation with increased expression or phosphorylation levels of differentiation and/or myelin marker proteins, suggesting the negative role of ACE2 in morphological differentiation. Notably, ACE2's intracellular region preferentially interacted with the active GTP-bound form of Ras. Thus, knockdown of ACE2 relatively increased GTP-bound Ras in an affinity-precipitation assay. Indeed, inhibition of Ras resulted in decreasing both morphological differentiation and expression or phosphorylation levels of marker proteins, confirming the positive role of Ras in differentiation. These results indicate the role of ACE2 itself as a negative regulator of oligodendroglial cell morphological differentiation, newly adding ACE2 to the list of regulators of oligodendroglial morphogenesis as well as of Ras-binding proteins. These findings might help us to understand why SARS-CoV-2 causes pathological effects in the CNS.
Keywords: ACE2; Ras; differentiation; oligodendrocyte; oligodendroglial cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

