The Role of Exposomes in the Pathophysiology of Autoimmune Diseases II: Pathogens
- PMID: 35736648
- PMCID: PMC9231084
- DOI: 10.3390/pathophysiology29020020
The Role of Exposomes in the Pathophysiology of Autoimmune Diseases II: Pathogens
Abstract
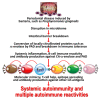
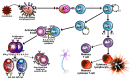
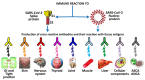
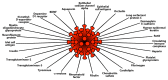
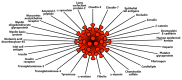
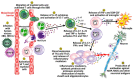
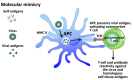
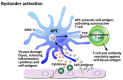
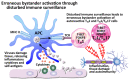
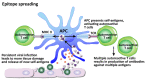
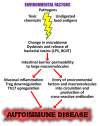
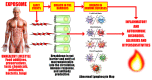
In our continuing examination of the role of exposomes in autoimmune disease, we use this review to focus on pathogens. Infections are major contributors to the pathophysiology of autoimmune diseases through various mechanisms, foremost being molecular mimicry, when the structural similarity between the pathogen and a human tissue antigen leads to autoimmune reactivity and even autoimmune disease. The three best examples of this are oral pathogens, SARS-CoV-2, and the herpesviruses. Oral pathogens reach the gut, disturb the microbiota, increase gut permeability, cause local inflammation, and generate autoantigens, leading to systemic inflammation, multiple autoimmune reactivities, and systemic autoimmunity. The COVID-19 pandemic put the spotlight on SARS-CoV-2, which has been called "the autoimmune virus." We explore in detail the evidence supporting this. We also describe how viruses, in particular herpesviruses, have a role in the induction of many different autoimmune diseases, detailing the various mechanisms involved. Lastly, we discuss the microbiome and the beneficial microbiota that populate it. We look at the role of the gut microbiome in autoimmune disorders, because of its role in regulating the immune system. Dysbiosis of the microbiota in the gut microbiome can lead to multiple autoimmune disorders. We conclude that understanding the precise roles and relationships shared by all these factors that comprise the exposome and identifying early events and root causes of these disorders can help us to develop more targeted therapeutic protocols for the management of this worldwide epidemic of autoimmunity.
Keywords: autoantibodies; autoimmunity; bystander activation; environmental trigger; epitope spreading; exposome; infection; molecular mimicry; pathogen; pathophysiology.
Conflict of interest statement
A.V. is co-owner, CEO, and technical director of Immunosciences Lab., Inc. E.V. is the owner and founder of Regenera Medical. A.Z.R. and Y.S. declare no conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
