Mesoamerican Cypripedium: Mycorrhizal Contributions to Promote Their Conservation as Critically Endangered Species
- PMID: 35736705
- PMCID: PMC9227847
- DOI: 10.3390/plants11121554
Mesoamerican Cypripedium: Mycorrhizal Contributions to Promote Their Conservation as Critically Endangered Species
Abstract
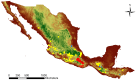
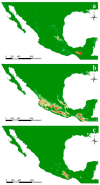
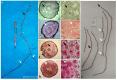
In the valuable orchid genus Cypripedium, the section Irapeana consists of a distinctive group of Mesoamerican species that is formed by Cypripedium dickinsonianum Hágsater, C. irapeanum Lex., and C. molle Lindl. All lady slipper orchids exhibit different distributions and abundances. Data analysis that used herbarium accessions and field investigations indicated that the habitats of these three species have been dramatically reduced. Prospecting for suitable habitats based on climatic, vegetation, and soil parameters allows us to predict potential distributions. Conservation strategies, such as ex situ propagation by asymbiotic and symbiotic approaches, have indicated that the culture media used are a determining factor for seedling development. Mycorrhizal isolates play a main role in the compatibility and further development of germinated seeds. The fungi isolated from adult plants belong to two different families, which makes it possible that widely distributed C. irapeanum populations will be fungal-specific as well as restricted for C. molle. Root mycorrhization patterns occur high on the secondary roots. In contrast with other species of the genus, in situ germination can occur over a short period of two months, but we have documented periods as long as ten years. Cypripedium is a highly problematic genus for ex situ conservation because the germination requirements and cultures are poorly documented, and there is great urgency for in situ conservation to develop strategies for identifying hotspot habitats and actualize the protection status to avoid extinction of this genus.
Keywords: habitat destruction; in vitro germination; orchid conservation; orchid mycorrhiza.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
First Guatemalan record of natural hybridisation between Neotropical species of the Lady's Slipper orchid (Orchidaceae, Cypripedioideae).PeerJ. 2017 Dec 22;5:e4162. doi: 10.7717/peerj.4162. eCollection 2017. PeerJ. 2017. PMID: 29302391 Free PMC article.
-
Non-specific symbiotic germination of Cynorkis purpurea (Thouars) Kraezl., a habitat-specific terrestrial orchid from the Central Highlands of Madagascar.Mycorrhiza. 2016 Aug;26(6):541-52. doi: 10.1007/s00572-016-0691-6. Epub 2016 Mar 17. Mycorrhiza. 2016. PMID: 26984810
-
Asymbiotic Seed Germination and In Vitro Seedling Development of the Endangered Orchid Species Cypripedium guttatum.Plants (Basel). 2023 Nov 7;12(22):3788. doi: 10.3390/plants12223788. Plants (Basel). 2023. PMID: 38005685 Free PMC article.
-
Seed biology and in vitro seed germination of Cypripedium.Crit Rev Biotechnol. 2014 Dec;34(4):358-71. doi: 10.3109/07388551.2013.841117. Epub 2013 Nov 6. Crit Rev Biotechnol. 2014. PMID: 24191720 Review.
-
Orchid Reintroduction Based on Seed Germination-Promoting Mycorrhizal Fungi Derived From Protocorms or Seedlings.Front Plant Sci. 2021 Jun 30;12:701152. doi: 10.3389/fpls.2021.701152. eCollection 2021. Front Plant Sci. 2021. PMID: 34276753 Free PMC article. Review.
Cited by
-
Isolation of Tulasnella spp. from Cultivated Paphiopedilum Orchids and Screening of Germination-Enhancing Fungi.J Fungi (Basel). 2023 May 23;9(6):597. doi: 10.3390/jof9060597. J Fungi (Basel). 2023. PMID: 37367533 Free PMC article.
References
-
- Chase M.W. Classification of Orchidaceae in the Age of DNA Data. Curtis’s Bot. Mag. 2005;22:2–7. doi: 10.1111/j.1355-4905.2005.00466.x. - DOI
-
- Bunch W.D., Cowden C.C., Wurzburger N., Shefferson R.P. Geography and soil chemistry drive the distribution of fungal associations in lady’s slipper orchid, Cypripedium acaule. Botany. 2013;91:850–856. doi: 10.1139/cjb-2013-0079. - DOI
-
- Cribb P.J. In: The Genus Cypripedium. 1st ed. Green P., editor. Timber Press; Portland, OR, USA: 1997.
-
- Gargiulo R., Pironon S., Zheleznaya E., Sanchez M.D., Balázs Z.R., Podar D., Wilkinson T., Jäkäläniemi A., Kull T., Väre H., et al. Phylogeography and post-glacial dynamics in the clonal-sexual orchid Cypripedium calceolus L. J. Biogeogr. 2019;46:526–538. doi: 10.1111/jbi.13528. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

