Human 2D Crypt Model for Assaying Intestinal Stem Cell Proliferation and Differentiation
- PMID: 35736812
- PMCID: PMC9337237
- DOI: 10.1021/acs.analchem.2c00905
Human 2D Crypt Model for Assaying Intestinal Stem Cell Proliferation and Differentiation
Abstract
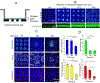
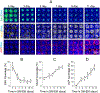
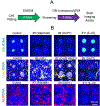
Intestine is a common site of adverse drug effects in clinical trials; thus, improved in vitro models for preclinical screening of pharmaceutical compounds are sought. A planar, self-renewing human intestinal monolayer platform based on primary adult gastrointestinal stem cells, termed the 2D crypt model, has been developed to screen for the effects of various compounds on the intestinal epithelium. The 2D crypt platform is based on a standard 12-well plate format and consists of cell culture inserts with a collagen film overlaying an impermeable film patterned with an array of micron-scale holes. This two-chamber format enables a gradient of growth factors to be applied such that the tissue self-organizes into spatially segregated stem and differentiated cell compartments. The patterned monolayer mimics a gut epithelium in possessing a stem cell niche, migrating proliferative and differentiated cells. Once established, the 2D crypts replicate many aspects of in vivo physiology, including cell migration, maturation, and apoptotic cell death. The planar geometry of the system simplifies dosing, sampling, and imaging during assay. An immunofluorescence-based assay was established to quantitatively assess cell density, proliferation, migration, viability, and the abundance and localization of postmitotic lineages as a function of time. The model was used to perform a small-scale screen of compounds, including signaling molecules, endogenous hormones/cytokines, and microbial metabolites, on tissue homeostasis. Hit compounds that significantly impacted proliferation and/or differentiation were readily identified. The 2D crypt platform represents a significant innovation in the development of microphysiological systems for emulating the gut epithelium for compound screens.
Conflict of interest statement
Y.W., C.E.S., and N.L.A. disclose a financial interest in Altis Biosystems, Inc.
Figures



References
-
- Koh A; De Vadder F; Kovatcheva-Datchary P; Bäckhed F From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. - PubMed
-
- Sung JH; Yu J; Luo D; Shuler ML; March JC Microscale 3-D hydrogel scaffold for biomimetic gastrointestinal (GI) tract model. Lab Chip 2011, 11, 389–392. - PubMed
-
- Meunier V; Bourrié M; Berger Y; Fabre G The human intestinal epithelial cell line Caco-2; pharmacological and pharmacokinetic applications. Cell Biol. Toxicol 1995, 11, 187–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

