Botulinum Toxin Type A for Treatment of Forehead Hyperhidrosis: Multicenter Clinical Experience and Review from Literature
- PMID: 35737033
- PMCID: PMC9231294
- DOI: 10.3390/toxins14060372
Botulinum Toxin Type A for Treatment of Forehead Hyperhidrosis: Multicenter Clinical Experience and Review from Literature
Abstract
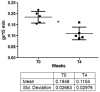
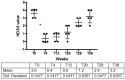
Among the forms of idiopathic hyperhidrosis, those involving the forehead have the greatest impact on patients' quality of life, as symptoms are not very controllable and are difficult to mask for patients. Although the local injection therapy with Incobotulinum toxin type A (IncoBTX-A therapy) can be considered a rational treatment, data from the literature describing both efficacy and safety of the treatment over the long term are poor. The aim of this report is to describe the single-center experience of five patients seeking treatment, for forehead hyperhidrosis with IncoBTX-A. To evaluate the benefits, safety profile and duration of anhidrosis, patients were treated following a standardized procedure and then followed until clinical relapse. The amount of sweating was measured by gravimetric testing, the extension of hyperhidrosis area was measured through Minor's iodine starch test, and response to the treatment was evaluated using the Hyperhidrosis Disease Severity Scale (HDSS) and the Dermatology Life Quality Index (DLQI). In all treated patients, a significant anhidrotic effect was observed 4 weeks after the treatment and lasted for approximately 36 weeks. The reduction in sweat production was associated with significant amelioration of symptoms and quality of life for all treated patients. No serious side effects occurred; one patient complained of a mild transient bilateral ptosis. Although further wider studies are required, our preliminary results seem to encourage the use of IncoBTX-A in forehead hyperhidrosis.
Keywords: DLQI; HDSS; Incobotulinum toxin type A; efficacy; forehead hyperhidrosis; gravimetric test; quality of life; safety.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

